Scroll to:
Sintering and thermokinetic modeling of the phase evolution in thermite powder mixtures under controlled heating
https://doi.org/10.17073/1997-308X-2025-5-5-16
Abstract
The behavior of compacted mixtures of metal powders (Al, Ti) and recycled metalworking wastes (Fe + Fe2O3 + C) during vacuum sintering under controlled heating was investigated to assess the possibility of producing in situ metal–matrix composites containing oxide strengthening particles. The starting materials were titanium and aluminum powders (particle size <160 μm and <100 μm, respectively) and a powder produced from recycled steel chips (<300 μm). The resulting samples exhibited a heterogeneous phase composition, which was examined by X-ray diffraction (CuKα radiation, XRD-6000 diffractometer) and optical microscopy (Axiovert 200MAT). A pronounced difference was observed between the aluminum- and titanium-based systems: the former exhibited a distinct thermal peak, whereas the latter showed smooth temperature behavior without thermal spikes. A thermokinetic model describing the multi-stage reactions in both systems was developed. The model incorporates metallothermic reduction and intermetallic formation reactions. Formal kinetic parameters were estimated using a semi-empirical approach and refined by comparison with experimental data. The governing equations, including the heat balance equation and the system of kinetic rate equations, were solved numerically using a semi-implicit Euler method, while mass conservation and atomic balance were verified. The initial composition of the samples was varied in the calculations – accounting for oxygen, carbon, and the Fe/Fe2O3 ratio in the steel-chippowder – to reproduce the experimentally observed product compositions. The calculated and experimental results showed qualitative agreement.
Keywords
For citations:
Knyazeva А.G., Korosteleva Е.N., Safronova V.S. Sintering and thermokinetic modeling of the phase evolution in thermite powder mixtures under controlled heating. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(5):5-16. https://doi.org/10.17073/1997-308X-2025-5-5-16
Introduction
The in-situ formation of strengthening particles in metal–matrix composites (MMCs) has become a focal point in powder-processing technologies owing to applications across the aerospace, automotive, and energy sectors, and it continues to attract sustained interest from diverse research groups [1–4]. The spectrum of MMCs obtainable by powder routes is broad – in terms of both matrix chemistries and sets of strengthening phases. Of particular interest are systems built from chemically reactive powder components, where reactions between constituents synthesize the strengthening phases in-situ as micron-scale inclusions. This is largely characteristic of combustion-synthesis routes that rely on metallothermic reactions [5–9]. Such reactions are typically strongly exothermic, which helps sustain the synthesis process. Creating the strengthening particles directly during composite fabrication is advantageous for interfacial bonding with the surrounding matrix. However, when a mixture permits metallothermic reduction (one metal reducing another), the presence of additional constituents can markedly redirect reaction pathways because local interaction conditions vary across different regions of the compact [10].
The problem becomes even less straightforward if one component of the powder mixture is itself a complex material – namely, a metallic base containing oxide inclusions – produced by recycling steel chips [11].
Here, the feasibility of producing metal–matrix composites from Al–Fe2O3–Fe and Ti–Fe2O3–Fe powder mixtures (optionally containing carbon) under sintering in a vacuum chamber is assessed, together with a theoretical description based on a thermokinetic model that accounts for the kinetics of the principal reactions.
1. Experimental
1.1. Materials and methods
Phase evolution under vacuum sintering with a controlled heating program (sintering temperature 1173–1473 K; 60-min hold) was examined for powder systems based on Ti–Al–Fe2O3/(Fe + C) using the following representative mixtures (from several possible combinations): Al + (Fe + Fe2O3 + C) and Ti + (Fe + Fe2O3 + C). Titanium powder TPP-8 (main fraction d < 160 μm) and aluminum powder PA-4 (d < 100 μm) were used to prepare the mixtures. As an analogue of the Fe + Fe2O3 + C composition, a powder produced from recycled steel 45 chips with a particle size not exceeding 300 μm was employed; its characteristics are described in detail in [11]. The component ratios in the mixtures were calculated to be sufficient both for metallothermic reduction and for intermetallic formation. The quantitative compositions of the powder mixtures under study are given in Table 1.
Table 1. Composition of the investigated powder mixtures, wt. %
|
Selection of proportions for the aluminum-containing mixtures was guided by the binary Al–Fe phase diagram. The first option corresponds to the α-AlFe3 field, while the second lies predominantly in the Al3Fe/AlFe phase region. Both options imply exothermic reactions.
The titanium-containing mixture comprised 75 wt. % Ti and 25 wt. % steel chips, which favors the formation of a matrix basis in the resulting composite as a solid solution of iron and oxygen in titanium.
Microstructural characterization was performed by optical microscopy, scanning electron microscopy (SEM), and X-ray diffraction (XRD). The following instruments were used: Axiovert 200MAT optical microscope (Carl Zeiss, Germany), Mira 3 LMU SEM (Tescan, Czech Republic), and XRD-600 (Shimadzu, Japan) and DRON-8 (Russia) diffractometers with CuKα radiation. Phase identification employed the PDF-4+ database and POWDER CELL 2.4 for full-profile analysis.
According to [11], chips oxidized in water and then milled form oxide-coated steel-chip fragments with an Fe core, containing dissolved carbon (≈1.5 wt. %). Although carbide phases were not detected by XRD, the presence of carbon in the steel-chip particles was confirmed by energy-dispersive X-ray spectroscopy (EDS). Carbon content, measured with a LECO ONH-836 gas-impurity analyzer (USA), showed a broad distribution (0.8–1.6 wt. %), reflecting the substantial heterogeneity of the processed steel-chip–derived particles. Their surfaces bear iron-oxide regions (Fe3O4/Fe2O3/FeO) [11], with the oxide fraction reaching ≈50–70 %. Because these oxide layers are highly nonuniform (Fig. 1), the contact between a chip-derived particle and Al or Ti powder depends on the local surface domain encountered. Within the same mixture, the second component can therefore contact metallic iron (carbon steel) and iron oxides simultaneously.
Fig. 1. Microstructure of the recycled steel 45 chips after oxidation and crushing |
1.2. Experimental results
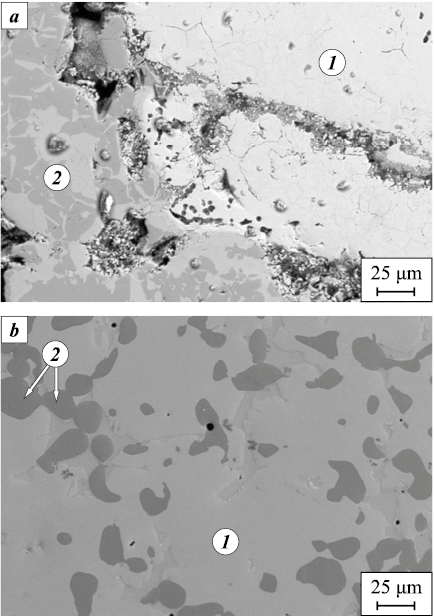
Under vacuum sintering at Ts = 1273 K, a heterogeneous phase assemblage was identified for both Al + (Fe + Fe2O3 + C) formulations [12]. The pressed compacts of aluminum powder with milled, oxidized steel chips lost structural integrity and disintegrated into separate fragments. The selected sintering conditions were sufficient to initiate a cascade of reactions, yielding Fe–Al intermetallics together with oxide strengthening particles. Residual (unreacted) iron was also detected in the products (Fig. 2). Oxygen was transferred almost entirely from the iron oxides to aluminum, forming Al2O3 , and FeAl constituted the dominant volumetric fraction of the product.
Fig. 2. Microstructure of a fragment of the sintered material |
With increasing aluminum content in the compact, the reaction products contained a substantial fraction of residual, unreacted constituents, attributable to a decrease in the overall exothermicity of the reactions.
The second formulation, Ti + (Fe + Fe2O3 + С), is less exothermic. Since the solubility of iron in β-Ti reaches 22 at. % at Ts = 1358 K and drops to 0.34 at. % in α-Ti at 673 K, it was expected that part of the iron would react with titanium to form intermetallics. It was also anticipated that titanium would be sufficient to interact with oxide inclusions present in the recycled steel-chip powder. Experimentally, the steel-chip powder in the Ti + (Fe + Fe2O3 + C) mixture exhibited good sinterability, with predominantly diffusional interaction with iron and oxygen migration from iron oxides into the titanium matrix. It is plausible that carbon contained in the chips enhances sintering via diffusion into titanium; however, its amount is too low to produce titanium carbide at levels detectable by X-ray diffraction. Interaction of free iron with titanium in the presence of oxide inclusions does not impede interdiffusion. The phase set and phase fractions depend on the sintering temperature; nevertheless, in all cases the products are dominated by a titanium-based solid solution (Fig. 3). At lower temperatures, the products contain a nonstoichiometric TiO2-based oxide (18 wt. %) and residual Fe2O3 (32 wt. %) originating from the steel-chip particles. Iron from these particles reacts with titanium to yield up to 20 wt. % of the equiatomic intermetallic TiFe. At a higher sintering temperature (1473 K), no unreacted constituents remain, and two types of α-Ti–based solid solutions form with different dissolved Fe and O contents; small inclusions of Ti2Fe, TiFe2 , or TiFe are also observed. Oxides are not detected as a separate phase under these conditions (Fig. 3).
Fig. 3. Microstructure of sintered compacts from the mixture |
The results reported in [13; 14] provided the basis for constructing thermokinetic models that capture the staged nature of phase formation.
2. Sintering model with detailed kinetics
2.1. Heat balance
The thermokinetic model accounts for the sample temperature change arising from the controlled external heating program and from heat release by chemical reactions. Owing to the small sample size, temperature gradients within the compact are neglected (a lumped-capacitance assumption); estimates justifying this approximation are given in [15]. The heat-balance equation has the form
| \[Vc\rho \frac{{dT}}{{dt}} = V{W_{ch}} + \sigma \varepsilon S\left( {T_W^4 - {T^4}} \right) - \alpha S\left( {T - {T_e}} \right),\] | (1) |
where T is the temperature; t is time; V and S are the compact volume and surface area; с and ρ are the heat capacity and density of the pressed powder mixture; Wch is the total heat release due to chemical reactions; σ is the Stefan–Boltzmann constant; ε is the surface emissivity of the compact; α is the heat-transfer coefficient (in vacuum it may be taken as zero); Те is the ambient temperature (if Newton cooling is included).
The vacuum-chamber wall temperature TW follows a linear law:
TW = T0 + at, for T < Ts , and TW = Ts , for T ≥ Ts , | (2) |
where а is the heating rate and Ts is the sintering temperature.
Within the interval Tmin–Tmax , melting is assumed to occur, with the liquid-phase fraction (ηL ) varying from 0 to 1:
| \[\begin{array}{c}{{\rm{\eta }}_{\rm{L}}} = {\rm{0} \rm{ }\rm{at}\rm{ }}T \le {T_{\min }},\\{{\rm{\eta }}_{\rm{L}}} = {\left( {\frac{{T - {T_{\min }}}}{{{T_{\max }} - {T_{\min }}}}} \right)^2}{\rm{ }\rm{at}\rm{ }}{T_{\min }} < T \le {T_{\max }},\\{{\rm{\eta }}_{\rm{L}}} = {\rm{1}\rm{ }\rm{at}\rm{ }}T > {T_{\max }}.\end{array}\] | (3) |
Here, Tmin is the lowest melting temperature among the reagents and reaction products in the chosen system, and Tmax is the highest one (see Table 2).
Table 2. Melting points of the starting substances
| |||||||||||||||||||||||||||||||||||
Within the melting interval, the (specific) heat capacity is given by
| \[c = \left( {{c_{\rm{S}}} + \frac{{{Q_{{\rm{eff}}}}}}{m}\frac{{\partial \eta }}{{\partial T}}} \right)\left( {1 - {\eta _{\rm{L}}}} \right) + {c_{\rm{L}}}{\eta _{\rm{L}}},\] | (4) |
where сS is the heat capacity of the mixture in the solid state, cL is the heat capacity of the mixture in the liquid state; Qeff is the effective enthalpy of fusion (J/mol); m is the mean molar mass of the mixture of reagents and reaction products.
Heat release from chemical reactions is given by
| \[{W_{{\rm{ch}}}} = \sum\limits_{i = 1}^n {{Q_i}{\Phi _i}} ,\] | (5) |
where n is the number of reactions; Φi are the reaction rates; Qi are the reaction enthalpies (heat effects).
2.2. Model of phase composition
evolution during sintering
Because the detailed mechanisms of most solid-state reactions are rarely known and the steps involved often comprise a mix of physicochemical processes, it is most appropriate to use a reduced-chemistry model that explicitly tracks the formation of the experimentally observed phases.
In the Al-containing system (where aluminum has the lowest melting temperature), the dominant exothermic step is expected to be the aluminothermic reduction of iron oxide:
| 2Al + Fe2O3 = Al2O3 + 2Fe. | (I) |
The reduced iron then reacts with excess aluminum to form intermetallic phases. Owing to the large reaction enthalpies, phase formation is expected to occur predominantly in the liquid phase and to be accompanied by the appearance of agglomerates [16]. At the same time, as noted in [17], direct reaction between aluminum and iron oxides during combustion is preceded by the partial decomposition sequence Fe2O3 → Fe3O4 → FeO. Thin plates of FeAl2O4 , have been observed in [17] as a result of the interaction between FeO and the amorphous Al2O3 film that invariably coats aluminum:
| FeO + Al2O3 → FeAl2O4 . | (II) |
Iron aluminates such as FeAl2O and FeAl2O4 commonly appear in the Al–Fe2O3 system, which is unfavorable for further reduction of iron oxides – especially in the presence of Al2O3 [18].
In the Al–Fe2O3 system, an additional step may occur
| 2Fe2O3 → 4FeO + O2 | (III) |
followed by oxidation of aluminum:
| 4Al + 3O2 = 2Al2O3 . | (IV) |
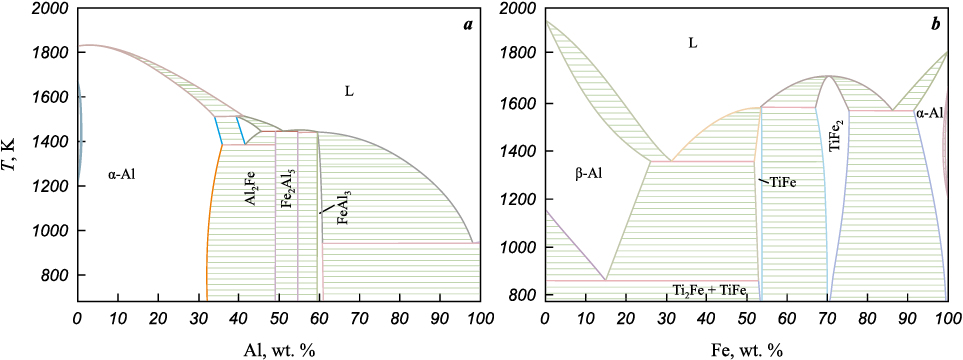
The Al–Fe phase diagram contains several intermetallic phases (Fig. 4). The diagram was constructed using the Thermo-Calc Software (open version) with the TCBIN: TC Binary Solutions v1.1 database.
Fig. 4. Phase diagrams of the Al–Fe (a) and Ti–Fe (b) systems |
Formation of intermetallic phases proceeds via the reactions
| Al + Fe = FeAl, | (V) |
| FeAl + Al = FeAl2 , | (VI) |
| FeAl + 2Fe = Fe3Al, | (VII) |
| FeAl2 + Al = FeAl3 , | (VIII) |
| FeAl2 + FeAl3 = Fe2Al5 . | (IX) |
The FeAl intermetallic is not shown on the diagram; however, it is known to form by ordering of the α-Fe(Al) solid solution. Fe3Al is a metastable phase that appears via a second-order phase transformation from FeAl [19]. Carbon present in the processed chips could, in principle, participate in the synthesis of aluminum carbide (Al4C3 ), but the sintering schedule and mixture composition do not provide sufficient carbon or temperature to initiate the corresponding reaction.
In the second case (Ti–Fe2O3–Fe–C system), iron is the lowest-melting component (see Table 2). According to [20], the thermite reaction Ti–Fe2O3 proceeds through several steps – reduction of Fe2O3 by Ti to form Fe and TiO2 , followed by the formation of the metastable intermetallic Ti2Fe. Allowing for partial decomposition of iron oxide, the presence of carbon, and the formation of intermetallic phases (Fig. 4, b), the reaction set can be written as:
| 3Ti + 2Fe2O3 = 3TiO2 + 4Fe, | (I′) |
| Ti + Fe = TiFe, | (II′) |
| TiFe + Ti = Ti2Fe, | (III′) |
| TiFe + Fe = TiFe2 , | (IV′) |
| Ti + C = TiC, | (V′) |
| 2Fe2O3 → 4FeO + O2 , | (VI′) |
| Ti + O2 = TiO2 , | (VII′) |
| TiO2 + Ti2Fe = Ti3FeO2 . | (VIII′) |
The notation for species concentrations (Сk ) for each system is given in Tables 3 and 4.
Table 3. Concentration designations for the Al + (Fe + Fe2O3 ) system
Table 4. Concentration designations for the Ti + (Fe2O3 + Fe + C) system
|
For each reaction network, the kinetic equations can be written as
| \[\frac{{d{C_k}}}{{dt}} = \sum\limits_{k = 1}^r {{\nu _{ki}}{\Phi _i}} ,\] | (6) |
where νki are the stoichiometric coefficients of component k in reaction i; r is the number of reactions, Фi are the reaction rates. We assume that the reaction rates follow an Arrhenius temperature dependence and depend on concentrations according to the law of mass action:
| \[{\Phi _i} = {k_i}(T)\prod\limits_k {C_k^{{n_{ki}}}} .\] | (7) |
Here, nki are the exponents equal in absolute value to the corresponding stoichiometric coefficients;
| \[{k_i} = {k_{i0}}\exp \left( { - \frac{{{E_i}}}{{RT}}} \right),\] | (8) |
where ki0 are the pre-exponential factors; Ei are the activation energies; R is the universal gas constant. The rate expressions for all reactions are given in Table 5.
Table 5. Reaction rate expressions
|
Thus, for the first system we require 27 formal kinetic parameters ki0 , Ei , Qi , and for the second – 24. Using published parameters for overall reactions in SHS-type mixtures [21] is not feasible. One reason is the strong dependence of parameters on the determination method, mixture processing, heating rate, etc. Another is the lack of data for most steps. Even for one of the most studied thermite mixtures (Al–Fe2O3 ), published values are inconsistent [22], including the reaction enthalpies. For example, for reaction (VI) in the Al-containing system, [23] gives: ΔH = –851.4 kJ/mol, whereas [16] reports ΔH ≈ –752 kJ/mol.
The entropies and enthalpies of reactions were obtained using Hess’s law:
| \[\Delta H_{298}^0 = \sum\limits_{{\rm{products}}} {{\nu _{ki}}\Delta H_{298}^0} - \sum\limits_{{\rm{reagents}}} {{\nu _{ki}}\Delta H_{298}^0} ,\] | (9) |
| \[\Delta S_{298}^0 = \sum\limits_{{\rm{products}}} {{\nu _{ki}}\Delta S_{298}^0} - \sum\limits_{{\rm{reagents}}} {{\nu _{ki}}\Delta S_{298}^0} ,\] | (10) |
where the first terms on the right-hand side are sums of the parameters for the reaction products, and the second terms are the parameters for the reactants (reagents in the formula). However, tabulated values are not available in the literature for all compounds. For this reason, approximate semi-empirical methods were used to obtain preliminary estimates of the formal kinetic parameters (Table 6). The parameter-estimation procedure is described in detail in [15].
Table 6. Formal kinetic parameters obtained using a semi-empirical approach
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For each system, the problem (which comprised 10 and 9 ordinary differential equations of the form (6) for the Al- and Ti-containing systems, respectively, plus the heat-balance equation (1)) was solved numerically using a semi-implicit Euler method. In every run, mass conservation and atomic balance were verified. Calculations were performed at constant \(\Delta S_{298}^0\) and \(\Delta H_{298}^0\). The reaction orders were adjusted during the numerical solution. For the first system, we obtained:
k01 = 1017, k02 = 1022, k03 = 3·1022, k04 = 1022,
k05 = 1022, k06 = 8·1019, k07 = 6·1015,
k08 = 1015, k09 = 1024 s–1.
For the second system, the adjustments affected two pre-exponential factors and the activation energy of one reaction:
k05 = 3·1019 s–1, k06 = 6·1023 s–1,
E6 = 150 255 J/mol.
A single scaling factor applied to all reactions was 10–17 for the Al-containing system and 7.5·10–13 for the Ti-containing system. The criterion for selecting this factor was the characteristic reaction time under the experimental conditions.
2.3. Numerical analysis results
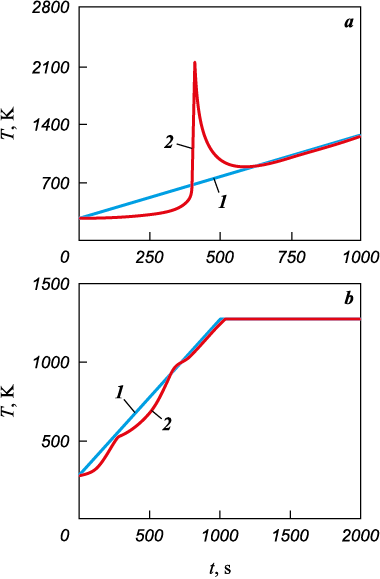
Calculated temperature and composition profiles for reactive sintering of the studied systems are shown in Figs. 5–7. The typical temperature curves differ for the Al- and Ti-containing mixtures (Fig. 5). In the former, a temperature spike appears upon reaction initiation at Т = 700–900 K. In the latter, there is no pronounced spike, although a wavy temperature curve is observed.
Fig. 5. Typical temperature curves for the composition with (a) and titanium (b)
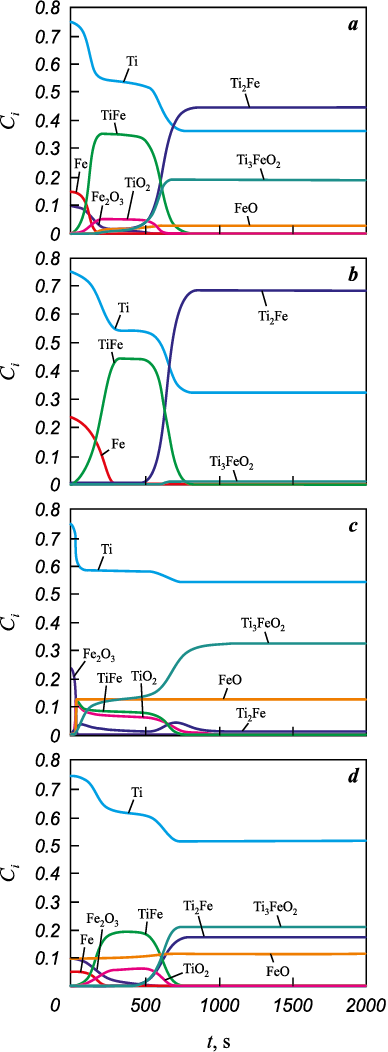
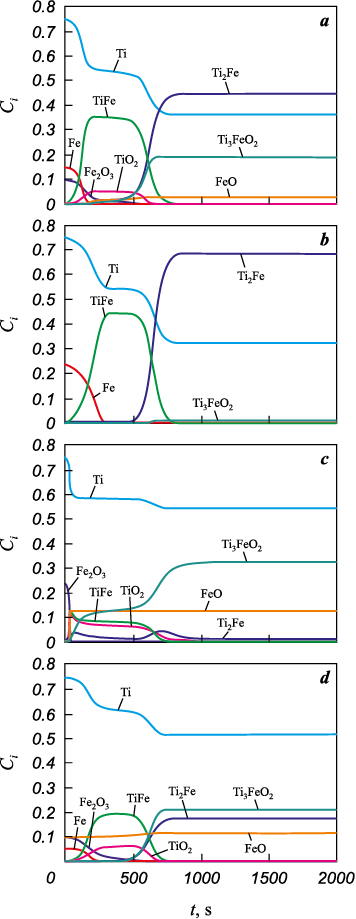
Fig. 6. Phase composition evolution
Fig. 7. Phase composition evolution |
Such behavior is often ascribed to thermocouple sensitivity; however, the calculations indicate that it may also arise from the interplay of coupled physicochemical processes.
As noted above, the exact oxide composition in the chips is unknown. Accordingly, the modeling allows for different choices of initial data (Table 7). We assume the presence of two iron oxides – FeO and Fe2O3 . Fe3O4 can, to first approximation, be treated as a combination of FeO and Fe2O3 and is therefore not included explicitly. In addition, the numerical experiment accounts for adsorbed oxygen and alumina on particle surfaces. Only under this assumption does the model reproduce the amount of Al2O3 observed experimentally in the products. The numerical study further enables exploration over a wide range of initial compositions, which is difficult to achieve experimentally.
Table 7. Initial data for calculations
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
No such complications arose for the Ti system, although the initial composition of the powder mixture can likewise be varied numerically.
Composition-evolution results for different initial data are presented in Figs. 6 and 7. We find that the product composition can vary substantially. For the Al-containing system (Fig. 6), the products always contain Fe3Al and FeAl3 intermetallics in different proportions, as well as Al2O3 . The reduction of iron is the fastest reaction. FeAl is not observed in the products. The mixed oxide appears only when the initial mixture contains Al2O3 , FeO, and oxygen; in that case, Fe2Al5 is also present. The experimental data are best matched by Composition 2, which includes oxygen and oxide in the starting mixture.
The phase composition of the Ti compact also evolves (Fig. 7). All reactions proceed actively over a time interval shorter than the total sintering time. For all initial compositions, the products always contain the intermetallic Ti2Fe and unreacted Ti. TiFe appears as an intermediate during sintering. In three variants, the product contains Ti3FeO2 . In the second variant, the starting mixture contains a small amount of iron oxide, hence its role is minor; however, Fe2O3 most closely reflects the experimental conditions. Under the experimental conditions, the product contains 91 wt. % of α-Ti solid solution + Ti2Fe and α-Ti solid solution + TiFe. In this case, Fe2O3 is insufficient to form TiO2 . In the other three variants, TiO2 appears as an intermediate that is consumed rapidly to form the complex oxide.
Conclusion
This study demonstrates good agreement between the proposed thermokinetic model and experimental data for multicomponent powder mixtures – recycled steel-chip powder with aluminum or titanium – under vacuum sintering. Consistent with the experiments, the calculations predict substantial heat release upon heating Al + (Fe + Fe2O3 + C) mixtures, whereas Ti + (Fe + Fe2O3 + C) sinters in a steady regime. In the former case, aluminum carbide was not included in the model because of the low sintering temperature; in the latter, titanium carbide formed only in trace amounts. No carbides were detected experimentally in either system.
References
1. Yeh C.L., Li R.F. Formation of TiAl–Ti5Si3 and TiAl–Al2O3 in situ composites by combustion synthesis. Intermetallics. 2008;16(1):64–70. https://doi.org/10.1016/j.intermet.2007.07.016
2. Zhang X., Li X., Wang J., Liu L., Li S., Li B., Hou X., Gao J., Kariya S., Umeda J., Kondoh K., Li S. Synthesis mechanism and interface contribution towards the strengthening effect of in-situ Ti5Si3 reinforced Al matrix composites. Materials Science and Engineering: A. 2024; 918:147427. https://doi.org/10.1016/j.msea.2024.147427
3. Yang Y.F., Mu D.K., Jiang Q.C. A simple route to fabricate TiC–TiB2/Ni composite via thermal explosion reaction assisted with external pressure in air. Materials Chemistry and Physics. 2014;143(2):480–485. https://doi.org/10.1016/j.matchemphys.2013.10.003
4. Lemboub S., Boudebane S., Gotor F.J., Haouli S., Mezrag S., Bouhedja S., Hesser G., Chadli H., Chouchane T. Core-rim structure formation in TiC–Ni based cermets fabricated by a combined thermal explosion/hot-pressing process. International Journal of Refractory Metals and Hard Materials. 2018;70:84–92. https://doi.org/10.1016/j.ijrmhm.2017.09.014
5. Yeh C.L., Ke C.Y. Synthesis of TiB2–Al2O3–FeAl composites via self-sustaining combustion with Fe2O3/TiO2-based thermite mixtures. Ceramics International. 2018;44(13):16030–16034. https://doi.org/10.1016/j.ceramint.2018.06.040
6. Zhang K., Fen W., Zhu J., Wu H. Mechanical properties and microstructure of Al2O3/TiAl in situ composites doped with Cr and V2O5. Science of Sintering. 2012;44(1):73–80. https://doi.org/10.2298/SOS1201073Z
7. Motlagh E.B., Nasiri H., Khaki J.V., Sabzevar M.H. Formation of metal matrix composite reinforced with nano sized Al2O3+Ni–Al intermetallics during coating of Al substrate via combustion synthesis. Surface & Coatings Technology. 2011;205(23-24):5515–5520. https://doi.org/10.1016/j.surfcoat.2011.06.026
8. Kim J.-W., Lee J.-M., Lee J.-H., Lee J.-C. Role of excess Al on the combustion reaction in the Al–TiO2–C system. Metals and Materials International. 2014;20(6): 1151–1156. https://doi.org/10.1007/s12540-014-6020-8
9. Miloserdov P.A., Gorshkov V.A., Andreev D.E., Yukhvid V.I., Miloserdova O.M., Golosova O.A. Metallothermic SHS of Al2O3–Cr2O3 + TiC ceramic composite material. Ceramics International. 2023;49(14):24071–24076. https://doi.org/10.1016/j.ceramint.2023.04.145
10. Korosteleva E.N., Knyazeva A.G., Nikolaev I.O. Phase formation in reactive sintering with reduction. Physical Mesomechanics. 2023;26(1):39–47. https://doi.org/10.1134/S1029959923010058
11. Korosteleva E.N., Nikolaev I.О. Evolution of the structural-phase state of steel swarf during its processing into a powdered product. Powder Metallurgy and Functional Coatings. 2024;18(4):6–16. https://doi.org/10.17073/1997-308X-2024-4-6-16
12. Korosteleva E.N., Nikolaev I.О., Baranovsky А.V. Synthesis and evolution of the structural and phase state of powder materials Al–Fe–Fe2O3 under heating conditions. In: Collection of materials of the XVII Minsk International Heat and Mass Transfer Forum (20–24 May 2024). Minsk: Institute of Heat and Mass Transfer n.a. Lykov of the NAS of Belarus, 2024. P. 746–748. (In Russ.).
13. Miedema A.R., de Chȃtel P.F, de Boer F.R., de Chatel P.F. Cohesion in alloys – fundamental of a semi-empirical model. Physica B + C. 1980;100(1):1–28. https://doi.org/10.1016/0378-4363(80)90054-6
14. Binnewies M., Milke E. Thermochemical data of elements and compounds. Wiley-VCH Verlag GmbH, 2008. 928 p. https://doi.org/10.1002/9783527618347
15. Safronova V.S., Knyazeva A.G., Korosteleva E.N. A theoretical and experimental study of phase formation in Ti–CuO powder mixtures under reactive sintering conditions. New Journal of Chemistry. 2025;49(3):893–909. https://doi.org/10.1039/d4nj03751k
16. Podergin V.А. Metallothermal systems. Moscow: Metallurgiya, 1992. 272 p. (In Russ.).
17. Korchagin M.A., Podergin V.A. Investigation of chemical transformations in the combustion of condensed systems. Combustion, Explosion, and Shock Waves. 1979;15(3):325–329. https://doi.org/10.1007/BF00785065
18. Liu W., Ismail M., Dunstan M.T., Hu W., Zhang Z., Fennell P.S., Scott S.A., Dennis J.S. Inhibiting the interaction between FeO and Al2O3 during chemical looping production of hydrogen. RSC Advances. 2015;5(3):1759–1771. https://doi.org/10.1039/C4RA11891J
19. Liu Y., Watanabe M., Okugawa M., Hagiwara T., Sato T., Seguchi Y., Adachi Y., Minamino Y., Koizumi Y. Resolving the long-standing discrepancy in Fe3Al ordering mobilities: A synergistic experimental and phase-field study. Acta Materialia. 2024;273:119958. https://doi.org/10.1016/j.actamat.2024.119958
20. Lin Y.-C., Shteinberg A.S., McGinn P.J., Mukasyan A.S. Kinetics study in Ti–Fe2O3 system by electro-thermal explosion method. International Journal of Thermal Sciences. 2014;84:369–378. https://doi.org/10.1016/j.ijthermalsci.2014.06.008
21. Mukasyan A.S., Shuck C.E. Kinetics of SHS reactions: A review. International Journal of Self-Propagating High-Temperature Synthesis. 2017;26(3):145–165. https://doi.org/10.3103/S1061386217030049
22. Durães L., Costa B.F.O., Santos R., Correia A., Campos J., Portugal A. Fe2O3/aluminum thermite reaction intermediate and final products characterization. Materials Science and Engineering: A. 2007;465(1-2):199–210. https://doi.org/10.1016/j.msea.2007.03.063
23. Bodaghi M., Zolfonoon H., Tahriri M., Karimi M. Synthesis and characterization of nanocrystalline α-Al2O3 using Al and Fe2O3 (hematite) through mechanical alloying. Solid State Sciences. 2009;11(2):496–500. https://doi.org/10.1016/j.solidstatesciences.2008.06.021
About the Authors
А. G. KnyazevaRussian Federation
Anna G. Knyazeva – Chief Researcher, Laboratory of Nonlinear Mechanics of Metamaterials and Multilevel Systems, Institute of Strength Physics and Materials Science
8/2 Akademicheskii Prosp., Tomsk 634055, Russia
Е. N. Korosteleva
Russian Federation
Elena N. Korosteleva – Senior Researcher, Laboratory of Physics of Powder Materials Consolidation
8/2 Akademicheskii Prosp., Tomsk 634055, Russia
V. S. Safronova
Russian Federation
Valeriya S. Safronova – Research Assistant, Laboratory of Nonlinear Mechanics of Metamaterials and Multilevel Systems
8/2 Akademicheskii Prosp., Tomsk 634055, Russia
Review
For citations:
Knyazeva А.G., Korosteleva Е.N., Safronova V.S. Sintering and thermokinetic modeling of the phase evolution in thermite powder mixtures under controlled heating. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(5):5-16. https://doi.org/10.17073/1997-308X-2025-5-5-16