Scroll to:
Synthesis of optically transparent YAG:Ru ceramics
https://doi.org/10.17073/1997-308X-2025-6-36-43
Abstract
Yttrium–aluminum garnet (YAG) ceramics doped with ruthenium atoms were synthesized in this study. The precursor powder was obtained by the coprecipitation method. The dopant, in the form of ruthenium (III) chloride, was introduced at different technological stages: during precursor powder synthesis and during deagglomeration of the ceramic powder, resulting in two series of samples. The phase composition of the sintered ceramics was examined by X-ray diffraction (XRD). According to the obtained data, no secondary or impurity phases were detected. Differential thermal analysis (DTA) revealed a decrease in the cationic homogeneity of the precursor powder. Incorporation of ruthenium into the YAG structure led to a shift of the exothermic crystallization peak toward higher temperatures. The ceramic samples were sintered at 1815 °C for 20 h, followed by annealing in air at 1500 °C for 2 h. Optical characterization of the ceramics showed that the method of dopant introduction affected both the optical transmittance and the band gap energy. The transmittance at 1100 nm for undoped YAG ceramics was 77.04 %, while for the ruthenium-containing samples it decreased to 65.1 and 74.5 %, depending on the dopant incorporation route. The band gap energy was determined from differential absorption spectra: for pure YAG it was 4.92 eV, and for the Ru-doped ceramics it decreased to a minimum of 4.4 eV.
Keywords
For citations:
Suprunchuk V.E., Kravtsov A.A., Lapin V.A., Malyavin F.F., Bedrakov D.P. Synthesis of optically transparent YAG:Ru ceramics. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(6):36-43. https://doi.org/10.17073/1997-308X-2025-6-36-43
Introduction
Yttrium–aluminum garnet (YAG) is a crystalline material with a cubic structure characterized by high thermal conductivity, chemical stability, and excellent physical and optical properties. These features make it suitable for a wide range of industrial applications. YAG is widely used in most laser systems [1], light-emitting diodes (LEDs), and various optical and electronic devices. It can be produced in both single-crystal and polycrystalline forms. Recently, polycrystalline materials doped with rare-earth elements have gained attention as alternatives to single crystals [2], the fabrication of which often poses challenges in achieving uniform dopant distribution [3].
In contrast to single crystals, ceramic processing allows not only for homogeneous atomic-level dopant distribution but also for the fabrication of components with controlled geometry and dimensions. Particular attention has been given to doping YAG with trivalent rare-earth ions [4]. The incorporation of impurity ions follows the general principles of ionic size and charge compatibility with the substituted garnet-forming ions [5]. It is well established that doping and variation of dopant concentration can alter the optical [6], mechanical, and thermal properties of the material [4; 7].
Ruthenium, a 4d-transition metal cation, is an attractive dopant owing to the diversity of its electronic states, which impart unique electronic, magnetic [8], photorefractive, and photochromic properties to the host matrix [9; 10]. Most studies on Ru applications focus on catalyst development [11; 12], conductive metallic coatings for electrochemical gas sensors [13], and chromatographic detectors [14]. In ceramic systems, ruthenium has been introduced into oxide matrices to enhance electronic conductivity [15; 16], dielectric permittivity [17], and electrical resistivity control [18], and to develop intermediate-temperature ion-transport ceramic membranes [19].
The behavior of Ru has been extensively studied in certain oxide systems, such as perovskite-type structures \(A{A'_3}{{\rm{B}}_4}{{\rm{O}}_{12}}\) [12; 16; 20]. However, no literature reports were found on the fabrication of optically transparent YAG:Ru ceramics. It can be assumed that the introduction of Ru into the YAG structure may enable targeted modification of its optical characteristics.
The present work aimed to obtain optically transparent YAG:Ru ceramics and to determine the optimal synthesis route. Our previous results demonstrated the feasibility of incorporating Ru into the garnet structure during ceramic powder synthesis [21]. Further study of YAG:Ru materials may reveal their potential for applications in the production of polycrystalline optical isolators, absorbers, and LEDs. Therefore, in this work, YAG:Ru compositions were synthesized using different methods of Ru incorporation into the YAG lattice, and the effects of Ru addition on the microstructural features, phase transformations of powders, phase composition, and optical properties of the final ceramics were investigated.
Materials and methods
The ceramic materials were synthesized using the following reagents:
– ammonia (25 %, pure grade, SigmaTek, Russia);
– aluminum chloride hexahydrate (99 %, Nevatorg, Russia);
– ruthenium (III) chloride (99 %, Anhui Herrman Impex Co. Ltd., China);
– yttrium chloride hexahydrate (99.9 %, Nevatorg, Russia);
– ammonium sulfate (99 %, Stavreakhim, Russia);
– isopropyl alcohol (99.7 %, Khimprom LLC, Russia);
– calcium chloride (99 %, Vekton, Russia);
– magnesium chloride (99.9 %, Interkhim, Russia).
Deionized water was used to prepare all solutions.
To determine the optimal stage for introducing the dopant, three types of samples were prepared: S_0 – pure YAG; S_Ru – YAG:Ru, where the dopant was added during precursor synthesis; S_0_Ru – YAG:Ru, where Ru was introduced during the deagglomeration of the ceramic powder in a ball mill.
The precursor powders S_0 and S_Ru were synthesized by the coprecipitation method. For this purpose, solutions of yttrium and aluminum salts (and additionally ruthenium salts for S_Ru) were added dropwise into a 2.7 % ammonia precipitant solution using a peristaltic pump. The salt solution also contained NH4(SO4)2 at a concentration of 0.08 M. The resulting precipitate was washed with 0.045 M ammonium sulfate solution, followed by isopropyl alcohol, and dried at 60 °C for 15 h. The dried precipitate was sieved through a 200-mesh screen, ground, and mixed with sintering additives. Grinding was carried out in a planetary ball mill (Pulverisette 5, Fritsch, Germany) using alumina balls (2 mm) for 30 min at 150 rpm in 0.2 M ammonium sulfate solution. The mass ratio of milling medium:grinding media:powder was 4.5:4.5:1.0. Magnesium oxide (MgO) and calcium oxide (CaO) were added as sintering aids at 0.1 at. % each. The powders were calcined in air at 1150 °C for 2 h in a high-temperature furnace (Nabertherm 40/17, Germany).
The S_0 powder was divided into two portions, and ruthenium (III) chloride was introduced into one of them. All powder samples were then ground in a planetary ball mill with alumina balls (1 mm) at a medium-to-ball-to-powder ratio of 3.5:5.5:1.0 for 20 min at 150 rpm. The resulting suspensions were dried and sieved through a 200-mesh screen. The powders were uniaxially pressed at 50 MPa and sintered under vacuum at 1815 °C for 20 h. The sintered samples were ground to a thickness of 2 ± 0.1 mm, polished using a QPol-250 setup, and annealed in air at 1500 °C for 2 h (Na.
The particle-size distribution was analyzed by laser diffraction (LDA) using a SALD-7500 nano analyzer (Shimadzu, Japan). The morphology of the powders and ceramics was studied by scanning electron microscopy (SEM) using a MIRA3-LMH microscope (Tescan, Czech Republic) equipped with an AZtecEnergy Standard/X-max 20 EDS system. The specific surface area was determined by the Brunauer–Emmett–Teller (BET) method on a 3Flex analyzer (Micromeritics, USA) by nitrogen adsorption at Т = 77 K.
Thermal behavior of the precursor powders was studied by differential thermal analysis (DTA) and thermogravimetry (TG) using an STA 449 F5 Jupiter analyzer (NETZSCH-Gerätebau GmbH, Germany) in the temperature range 20–1300 °C under air flow (25 °C/min). Phase composition of the ceramics was examined by X-ray diffraction (XRD) on a TD-3700 diffractometer (Tongda, China) equipped with a CuKα radiation source (λ = 1.5406 Å).
Optical transmittance in the wavelength range λ = 200–1100 nm was measured using an SF-56 spectrophotometer (OKB-Spektr, Russia).
Results and discussion
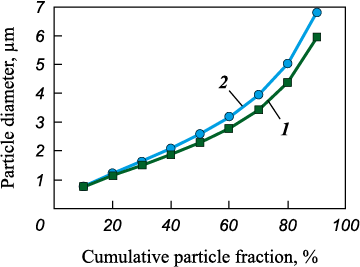
At the first stage, YAG and YAG:Ru precursor powders were synthesized and characterized in terms of particle-size distribution. Fig. 1 shows the cumulative particle-size curves of both powders. In both cases, a monomodal distribution with similar values was observed. The median particle diameters (d50 ) for S_0 and S_Ru were 2.3 and 2.6 μm, respectively, indicating a negligible effect of cationic composition on powder dispersion.
Fig. 1. Cumulative particle-size distribution curves |
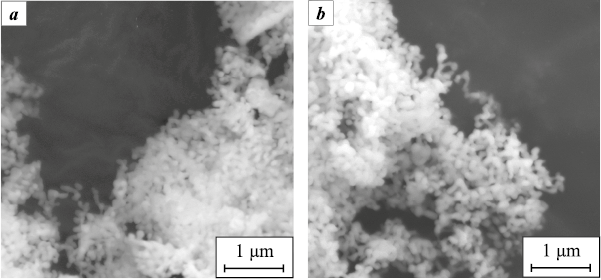
The morphology of YAG and YAG:Ru powders was examined by SEM. As shown in Fig. 2, the particles form loose, coarse agglomerates. They have an elongated shape and may consist of several crystallites connected by necks. No morphological differences were observed between S_0 and S_Ru powders.
Fig. 2. SEM images of ceramic powders S_0 (a) and S_Ru (b) |
The specific surface areas were also comparable – 11.06 m2/g for YAG and 10.28 m2/g for YAG:Ru – indicating a branched surface structure. Thus, the introduction of Ru did not significantly affect the morphology of the ceramic powders.
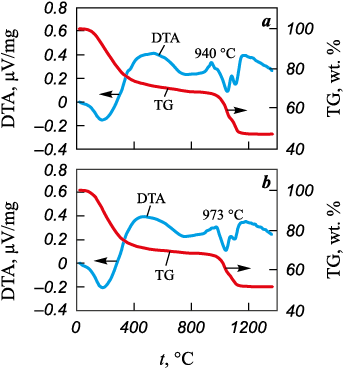
Thermal analysis curves of precursor powders S_0 and S_Ru are shown in Fig. 3. Both samples exhibited pronounced weight loss. The first critical weight-loss region (≈30 %) occurred between 100 and 450 °C and was attributed to the removal of adsorbed and chemically bound water, ammonia, and nitro groups [22; 23], as indicated by the endothermic peak at 200 °C. The second weight-loss region (900–1100 °C) included two endothermic and one exothermic peak. The endothermic peaks correspond to the decomposition of sulfates and oxysulfates and desorption of sulfate groups [24], while the exothermic peak near 940 °C is associated with YAG crystallization [25]. In the Ru-doped sample, this crystallization peak shifted toward higher temperatures, likely due to reduced cationic homogeneity of the precursor powder. Broadening of this peak suggests the formation of intermediate phases prior to YAG crystallization.
Fig. 3. Thermogravimetric (TGA) and differential thermal analysis (DTA) |
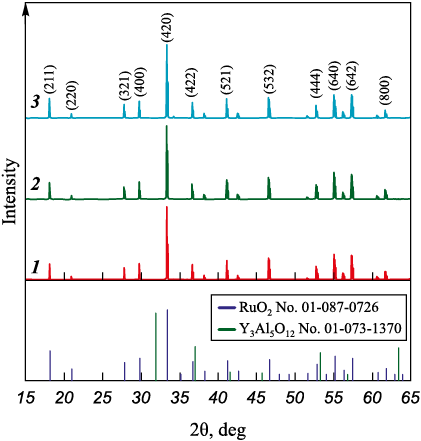
The phase composition of the ceramics after vacuum sintering at 1815 °C for 20 h was determined for S_0, S_Ru, and S_0 _Ru samples. XRD patterns (Fig. 4) confirmed that all samples were single-phase solid solutions with a garnet structure and contained no secondary or Ru-bearing impurity phases such as RuO2 . This indicates structural uniformity of the synthesized YAG ceramics regardless of the Ru introduction method.
Fig. 4. XRD patterns of YAG ceramics |
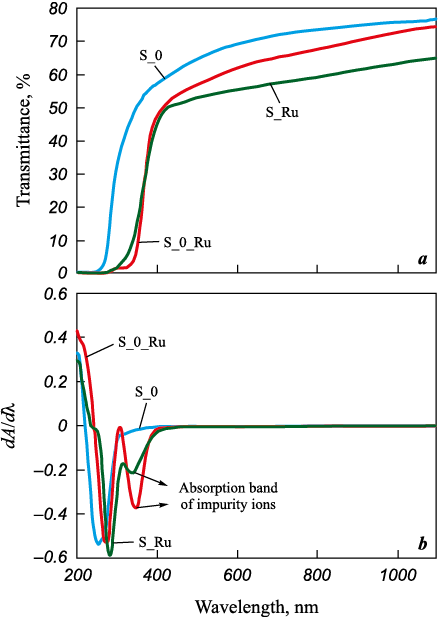
Optical characterization of the ceramics was then carried out. Prior to measurement, all samples were annealed in air at 1500 °C for 2 h. The transmittance spectra (Fig. 5, a) revealed that the linear optical transmittance at 1100 nm was 77.04 % for YAG, 65.1 % for S_Ru, and 74.5 % for S_0_Ru. These results indicate that Ru doping decreases the optical transparency of YAG, particularly when Ru is introduced during hydroxide precipitation.
Fig. 5. Optical transmittance spectra (a) |
Reduced transmittance was observed across the entire wavelength range (200–1100 nm), which may be attributed to color changes induced by Ru incorporation into the garnet lattice (Fig. 6). The gray coloration likely results from the formation of oxygen vacancies that act as color centers due to electron association and remain partially stable after air annealing [26]. Therefore, the observed coloration is directly related to the dopant ions introduced.
Fig. 6. Photographs of ceramic samples S_0, S_Ru, and S_0_Ru |
Additionally, a shift in the absorption edge was observed, which can be associated with lattice disorder caused by Ru doping and a decrease in the band gap energy. The latter was calculated from the absorption spectra derived from transmittance data [27] and differentiated (Fig. 5, b). The differential absorption spectrum (rate of absorbance change dA/dλ) of pure YAG exhibits a single absorption edge corresponding to a band gap of 4.92 eV. For the S_Ru and S_0_Ru samples, two absorption edges were observed, likely due to intrinsic absorption of ruthenium ions through Ru3+ → Ru4+ transitions. The calculated band gap energies for S_Ru and S_0_Ru were 4.4 and 4.54 eV, respectively.
Thus, the sample doped during the deagglomeration stage exhibited higher optical transmittance and a smaller absorption-edge shift, indicating that this method provides the most favorable route for obtaining optically transparent YAG:Ru ceramics.
Conclusions
YAG:Ru ceramic powders were synthesized by the coprecipitation method, and the optimal stage for introducing ruthenium (III) chloride into the system was identified. According to DTA, incorporation of Ru into the garnet lattice shifts the exothermic YAG formation peak to higher temperatures, which is consistent with reduced cation homogeneity in the YAG precursor. Ru in the garnet structure also decreases optical transmittance across the entire measured wavelength range (200–1100 nm): from 77.04 % for undoped YAG to a minimum of 65.1 % for YAG:Ru.
Ceramics obtained when the dopant was introduced during deagglomeration of the ceramic powder exhibited a higher linear transmittance (74.5 %) and a smaller absorption-edge shift – with the band gap decreasing from 4.92 eV (pure YAG) to 4.54 eV (YAG:Ru) – which makes this route the preferred method for producing optically transparent YAG:Ru ceramics.
Although Ru doping lowers transmittance, it modulates the optical response relative to pure YAG. The combined effects – band-gap narrowing and enhanced absorption – are promising for the development of broadband absorbers, neutral-density optical filters, and passive optical limiting devices.
References
1. Ikesue A., Aung Y.L., Taira T., Kamimura T., Yoshida K., Messing G.L., Progress in ceramic lasers. Annual Review of Materials Research. 2006;36:397–429. https://doi.org/10.1146/annurev.matsci.36.011205.152926
2. Ali H., Masschelein P., Bruyere S., Pigeat P., Dauscher A., Rinnert H., Horwat D., Khedr M.A., Giba A.E. White light emission from Sm-doped YAG ceramic controlled by the excitation wavelengths. Optics & Laser Technology. 2021;142:107223. https://doi.org/10.1016/j.optlastec.2021.107223
3. Lu J., Prabhu M., Song J., Li C., Xu J., Ueda K., Kaminskii A.A., Yagi H., Yanagitani T. Optical properties and highly efficient laser oscillation of Nd:YAG ceramics. Applied Physics B: Lasers and Optics. 2000;71(4):469–473. https://doi.org/10.1007/s003400000394
4. Timoshenko A.D., Matvienko O.O., Doroshenko A.G., Parkhomenko S.V., Vorona I.O., Kryzhanovska O.S., Safronova N.A., Vovk O.O., Tolmachev А.V., Baumer V.N., Matolínová I., Hau S., Gheorghe C., Yavetskiy R.P. Highly-doped YAG:Sm3+ transparent ceramics: Effect of Sm3+ ions concentration. Ceramics International. 2023;49(5):7524–7533. https://doi.org/10.1016/j.ceramint.2022.10.257
5. Boulesteix R., Maître A., Baumard J.-F., Sallé C., Rabinovitch Y. Mechanism of the liquid-phase sintering for Nd:YAG ceramics. Optical Materials. 2009;31(5): 711–715. https://doi.org/10.1016/j.optmat.2008.04.005
6. Wu X., Wang S., Wong-Ng W., Gu Q., Jiang Y., Wang C., Ma S., Liu W. Novel optical properties and induced magnetic moments in Ru-doped hybrid improper ferroelectric Ca3Ti2O7 . Journal of Advanced Ceramics. 2021;28;10(1):120–128. https://doi.org/10.1007/s40145-020-0425-2
7. Yagi H., Yanagitani T., Numazawa T., Ueda K. The physical properties of transparent Y3Al5O12 . Ceramics International. 2007;33(5):711–714. https://doi.org/10.1016/j.ceramint.2005.12.007
8. Banerjee A. Fluoride electrolyte based galvanic cell: Stability of the hollandite BaRu6O12(s). Journal of Fluorine Chemistry. 2021;245:109779. https://doi.org/10.1016/j.jfluchem.2021.109779
9. Wu S.-Y., Fu Q., Lin J.-Z., Zhang H.-M. Theoretical studies of the local structures and the EPR parameters for Ru3+ in the garnets. Optical Materials. 2007;29(8): 1014–1018. https://doi.org/10.1016/j.optmat.2006.03.036
10. Chiang C.H., Chen J., Hu C. Photorefractive and photochromic properties of Ru-doped lithium niobate crystal. In: Proc. of Conference on Lasers and Electro-Optics Europe-Technical Digest. Munich, Germany, 2007. CC_15. https://doi.org/10.1109/CLEOE-IQEC.2007.4386031
11. Masuda Y., Hosokawa S., Inoue M. Combustion activities of the Ru catalysts supported on hexagonal YbFeO3 . Journal of the Ceramic Society of Japan. 2011; 119(1395):850–854. https://doi.org/10.2109/jcersj2.119.850
12. Sun N., Li W., Qin Y., Zheng Z., Zhang B., Dong X., Wei P., Zhang Y., He X., Xie X., Huang K., Wu L., Lei M., Gou H., Yu R. Screening A-site ordered quadruple perovskites for alkaline hydrogen evolution reaction via unifying electronic configuration descriptor. Chinese Physics B. 2024;33(12):128101. https://doi.org/10.1088/1674-1056/ad8074
13. Fesik E.V., Grebnev V.V., Zarazhevskii V.I., Mal’chikov G.D. Ruthenium coatings on zirconium dioxide ceramic: Physicochemical and functional properties. Russian Journal of Applied Chemistry. 2014;87(5):591–595. https://doi.org/10.1134/S1070427214050097
14. Salimi A., Pourbeyram S., Amini M.K. Renewable-surface sol–gel derived carbon ceramic electrode fabricated by [Ru(bpy)(tpy)Cl]PF 6 and its application as an amperometric sensor for sulfide and sulfur oxoanions. The Analyst. 2002;127(12):1649–1656. https://doi.org/10.1039/B209194A
15. Hu D., Wang R., Du P., Li G., Wang Y., Fan D., Pan X. Electrospinning Ru doped Co3O4 porous nanofibers as promising bifunctional catalysts for oxygen evolution and oxygen reduction reactions. Ceramics International. 2022;48(5):6549–6555. https://doi.org/10.1016/j.ceramint.2021.11.202
16. Veselinović L., Mitrić M., Mančić L., Jardim P.M., Škapin S.D., Cvjetićanin N., Milović M.D., Marković S. Crystal Structure and Electrical Properties of Ruthenium-Substituted Calcium Copper Titanate. Materials. 2022;29;15(23):8500. https://doi.org/10.3390/ma15238500
17. Li W., Zhang T., Liu S., Lu Z., Xiong R. Decrease in the dielectric loss of CaCu3Ti4O12 at high frequency by Ru doping. Ceramics International. 2017;43(5):4366–4371. https://doi.org/10.1016/j.ceramint.2016.12.082
18. Annamalai S., Vidensky I., Pegg I.L., Dutta B. Effect of cation stoichiometry on the transport properties of calcium ruthenium oxide ceramics. Journal of Materials Science. 2008;43(14):4996–5004. https://doi.org/10.1007/s10853-008-2739-2
19. Dergacheva P.E., Kulbakin I.V., Fedorov S.V., Lysenkov A.S., Artemov V.V. Ceramic сomposite membranes based on Bi3Ru3O11–Bi1.6Er0.4O3 for obtaining of oxygen. Inorganic Materials: Applied Research. 2021;12(5):1326–1331. https://doi.org/10.1134/S2075113321050087
20. Mizumaki M., Mizokawa T., Agui A., Tanaka S., Takatsu H., Yonezawa S., Maeno Y. Oxygen hole state in A-site ordered perovskite ACu3Ru4O12 (A = Na, Ca, and La) probed by resonant X-ray emission spectroscopy. Journal of the Physical Society of Japan. 2013;82(2):024709. https://doi.org/10.7566/JPSJ.82.024709
21. Suprunchuk V.E., Kravtsov A.A., Tarala L.V., Medyanik E.V., Malyavin F.F., Lapin V.A., Bedrakov D.P. Synthesis of yttrium aluminum garnet ceramic powder doped with ruthenium. Fiziko-khimicheskie aspekty izucheniya klasterov, nanostruktur i nanomaterialov. 2024;(16):1016–1024. (In Russ.). https://doi.org/10.26456/pcascnn/2024.16.1016
22. Kravtsov A.A., Nikova M.S., Vakalov D.S., Tarala V.A., Chikulina I.S., Malyavin F.F., Chapura O.M., Krandievsky S.O., Kuleshov D.S., Lapin V.A. Combined effect of MgO sintering additive and stoichiometry deviation on YAG crystal lattice defects. Ceramics International. 2019;45(16):20178–20188. https://doi.org/10.1016/j.ceramint.2019.06.287
23. Jing W. , Li F., Yu S., Ji X., Xu T., Zhang J., Pan Z., Yuan Z., Kang B., Deng J., Yin W., Huang H. High efficiency synthesis of Nd:YAG powder by a spray co-precipitation method for transparent ceramics. Journal of the European Ceramic Society. 2018;38(5):2454–2461. https://doi.org/10.1016/j.jeurceramsoc.2017.12.059
24. Kravtsov A.A., Chapura O.M., Tarala V.A., Medyanik E.V., Tarala L.V., Suprunchuk V.E., Malyavin F.F., Kuznetsov S.V., Tsvetkov V.S., Dobretsova E.A., Kalachev Y.L., Lapin V.A. Fabrication and characterization of LuAG: Er ceramics with high optical transmission. Journal of the European Ceramic Society. 2025;45(3):117033. https://doi.org/10.1016/j.jeurceramsoc.2024.117033
25. Ru Y., Jie Q., Min L., Guoqiang L. Synthesis of yttrium aluminum garnet (YAG) powder by homogeneous precipitation combined with supercritical carbon dioxide or ethanol fluid drying. Journal of the European Ceramic Society. 2008;28(15):2903–2914. https://doi.org/10.1016/j.jeurceramsoc.2008.05.005
26. Zhang W., Lu T., Wei N., Ma B., Li F., Lu Z., Qi J. Effect of annealing on the optical properties of Nd:YAG transparent ceramics. Optical Materials. 2012;34(4):685–690. https://doi.org/10.1016/j.optmat.2011.10.001
27. Huang C.H., Zhang G., Chen Z.Q., Huang X.J., Shen H.Y. Calculation of the absorption coefficients of optical materials by measuring the transmissivities and refractive indices. Optics & Laser Technology. 2002;34(3):209–211. https://doi.org/10.1016/S0030-3992(01)00112-8
About the Authors
V. E. SuprunchukRussian Federation
Viktoria E. Suprunchuk – Cand. Sci. (Chem.), Associate Professor, Senior Researcher, Sector of Nanopowder for Synthesis, Research Laboratory Advanced Materials and Laser Media (RL AMLM)
1a Pushkin Str., Stavropol 355000, Russia
A. A. Kravtsov
Russian Federation
Alexander A. Kravtsov – Cand. Sci. (Eng.), Head, Sector of Nanopowder Synthesis, RL AMLM
1a Pushkin Str., Stavropol 355000, Russia
V. A. Lapin
Russian Federation
Vyacheslav A. Lapin – Cand. Sci. (Eng.), Senior Researcher, Sector of Physicochemical Methods of Analysis, RL AMLM
1a Pushkin Str., Stavropol 355000, Russia
F. F. Malyavin
Russian Federation
Fedor F. Malyavin – Head, Ceramics Sintering Sector, RL AMLM
1a Pushkin Str., Stavropol 355000, Russia
D. P. Bedrakov
Russian Federation
Dmitry P. Bedrakov – Engineer, Operations and Maintenance Sector, RL AMLM
1a Pushkin Str., Stavropol 355000, Russia
Review
For citations:
Suprunchuk V.E., Kravtsov A.A., Lapin V.A., Malyavin F.F., Bedrakov D.P. Synthesis of optically transparent YAG:Ru ceramics. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(6):36-43. https://doi.org/10.17073/1997-308X-2025-6-36-43