Scroll to:
Microstructure and phase composition of hard alloys produced from nanocrystalline powder mixture WC-6wt.%Co with C, Al and ZrC additives
https://doi.org/10.17073/1997-308X-2023-1-49-62
Abstract
A large specific surface area of WC nanopowder determines its high chemical activity and makes it very sensitive to various impurities, among which oxygen is most harmful and unavoidable. During heating, oxygen interacts with carbon of WC being removed in the form of CO/CO2, which finally leads to the appearance of embrittling η-phases in the hard alloy, abnormal growth of WC grains, and formation of a porous microstructure. To prevent heavy decarburization of WC during vacuum sintering of hard alloy from a nanocrystalline powder mixture WC-6wt.%Co, in this work we compared three methods: addition of extra carbon to compensate for carbon loss as a result of decarburization; addition of Al to bind impurity oxygen into Al2O3 before it interacts with carbon of WC; and addition of ZrC to compensate for carbon loss and bind impurity oxygen into ZrO2. Nanocrystalline powder mixtures based on WC-6 wt.%Co with and without additions of C, Al, and ZrC were prepared from microcrystalline powders of WC, Co, Al, ZrC, and carbon black by high-energy milling, then they were compacted in a cylindrical mold by uniaxial pressing at a pressure of ~460 MPa and sintered in graphite crucibles for 15 min at 1380 °C in vacuum of ~10-2 Pa. The heating rate to the temperature of sintering was 10 °C/min. The initial powders, powder mixtures prepared therefrom, and sintered hard alloys were certified using X-ray diffraction, chemical analysis, scanning electron microscopy, BET adsorption method, helium pycnometry, and Vickers method. The studies performed showed that the average particle size in all the prepared powder mixtures does not exceed 100 nm, and the content of impurity oxygen in them varies from 3.3 to 4.3 wt.% depending on the additives. It was established that only a part of oxygen contained in the powder mixtures is in the chemisorbed state and takes part in the decarburization of WC during vacuum sintering. The Al additive is completely oxidized during milling of the powder mixture and transforms into nanocrystalline Al2O3, which only aggravates carbon loss during sintering and results in the formation of a multiphase and relatively porous microstructure of the hard alloy. On the contrary, using carbon and ZrC additives we managed to prevent the decarburization of WC during sintering of the hard alloy and to form a less porous microstructure in it. It was shown that the presence of ZrO2 inclusions does not impede intensive growth of WC grains during sintering, but rather promotes it. Carbon deficit slightly suppresses intensive WC grain growth during sintering of hard alloy leading to the formation of η-phases and to an increase in the density and microhardness, but the presence of oxide inclusions Al2O3 and ZrO2 in the microstructure reduces the values of these properties.
Keywords
For citations:
Briakunov S.V., Kurlov A.S. Microstructure and phase composition of hard alloys produced from nanocrystalline powder mixture WC-6wt.%Co with C, Al and ZrC additives. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(1):49-62. https://doi.org/10.17073/1997-308X-2023-1-49-62
Introduction
Thanks to an outstanding combination of high hardness values and impact toughness, hard alloys, compared to other cutting materials (such as diamond or high speed steels), have a wide range of applications in many industries, e.g. as cutting tools (turning, milling, drilling tools) for metalworking, as part of the components of drill bits for well drilling, tunneling, and road pavement removal, as wear-resistant parts in drawing and stamping tools, etc. [1–3].
In the vast variety of available hard alloys, WC–Co system alloys are among the most common and required. The combination of the high hardness and strength of WC, which is maintained even at relatively high temperatures, with the ductility and high impact toughness of Co results in WC–Co alloys with high hardness, strength and wear resistance [4; 5]. Research into the physical, mechanical and performance properties of these alloys continues to this day. In the last three decades, the main efforts were aimed at developing various methods of obtaining nanocrystalline WС powders and mixtures based on them [6–10], as well as methods of their consolidation [11–20] for the production of hard alloys with a submicro- and nanocrystalline structure, which would enable significant improvement of their mechanical properties [21–23].
However, the transition from the use of microcrystalline to nanocrystalline carbide powders also exacerbates their contamination problem. The extremely large specific surface area of nanopowders determines their high chemical activity and makes them very sensitive to various impurities. The surface of carbide nanoparticles may contain adsorbed water and other impurities. Oxygen is the most harmful and unavoidable of these contaminants, its content usually greatly exceeding the total amount of all other impurities and determining the overall purity of the carbide nanopowder [24]. It was shown in [25; 26] that vacuum heating of nanocrystalline WC powders, regardless of their production method, is accompanied by decarburization of WC and leads to a change in their phase composition. When carbon is added to WC nanopowder, the latter retains its single-phase nature, but the strong growth of carbide particles is provoked, transforming the powder into a microcrystalline one. In the case of WC–Co hard alloys produced from nanopowders, decarburization during sintering caused by oxygen adsorbed on the surface of nanoparticles ultimately leads to the formation of embrittling η-phases in the alloy and abnormal growth of carbide grains [27–29].
The study of micro- and nanocrystalline TaC powders has shown that the content of adsorbed oxygen in them increases linearly with the specific surface area of the powder and that most of the oxygen in the powders is predominantly in the chemisorbed state, forming several monolayers of the Ta2O5 oxide phase on the particle surface [30]. An assessment of the possible loss of carbide carbon due to the desorption of chemisorbed oxygen in the form of CO showed that high-temperature sintering of nanocrystalline TaC powders, in contrast to microcrystalline ones, can be accompanied by their significant decarburization, which ultimately leads not only to a change in the composition (y) of TaCy carbide, but also to a change in the phase composition of the entire powder, which was later confirmed experimentally [31]. Besides, the desorption of chemisorbed oxygen in the form of CO and CO2 during sintering of dense compacts of carbide nanopowders results in the formation of a porous structure [32]. To avoid this, the impurity oxygen must be bound during sintering into strong, hard and refractory oxides that take the place of possible pores before the oxygen begins to interact with the carbon of the carbide. Candidates for this role may be Al or Zr, which have a higher affinity for oxygen compared to W and form the oxides Al2O3 and ZrO2 that are well known as the basis of modern ceramic materials with high mechanical strength, hardness, wear resistance, refractoriness, chemical and corrosion resistance [33; 34].
As evidenced by numerous publications [35–38], the practice of modifying WC–Co hard alloys with Al2O3 or ZrO2 nanoparticles to improve their physical and mechanical characteristics and performance has been in place for a long time. However, to form these particles during sintering, oxide nanoparticles, rather than pure metals, are added to WC-based nanocrystalline powder mixtures, as a rule. There are studies on the effect of Al additives in WC–Co powder mixtures, but they are usually microcrystalline powders with a low content of impurity oxygen; therefore, no Al2O3 is formed after sintering and only the presence of intermetallic Al–Co phases is detected [39].
The aim of this study is to find out whether it is possible to prevent strong decarburization of WC in a compacted WC–Co nanocrystalline powder mixture with the help of Al, ZrC, and carbon additives during conventional vacuum sintering and how these additives affect the microstructure and microhardness of the hard alloy.
Materials and Methods
To compensate for the loss of carbon and prevent strong decarburization of WC during vacuum sintering of a hard alloy, the results of using three additives were compared: carbon – to compensate for losses due to decarburization; aluminum – for binding impurity oxygen into solid and refractory oxide Al2O3 before its interaction with the carbon of WC; ZrC – to compensate for the loss of carbon and binding of impurity oxygen into refractory oxide ZrO2 .
The selected additives were introduced into the powder mixture in different amounts, due to the sequence of the experiments and the results obtained. The addition of Al was calculated based on the loss of carbon, which was determined from the change in the phase composition during the sintering of the WC nanopowder (without Co). The sample sintered from the WC nanopowder contained, along with WC, about 7.5 wt. %1 W2C, which corresponds to ~0.2 % carbon deficiency (loss) for the formation of single-phase WC. Assuming that the loss of carbon occurred only as a result of interaction with adsorbed oxygen with the formation of CO, at least 0.3 % of oxygen would be required to remove 0.2 % of carbon. To bind 0.3 % oxygen into Al2O3 oxide, a minimum of 0.4 % Al is required, taking into account that there is always an oxide film on the surface of Al particles the thickness of which in nanopowders obtained by milling is ~5 nm [40].
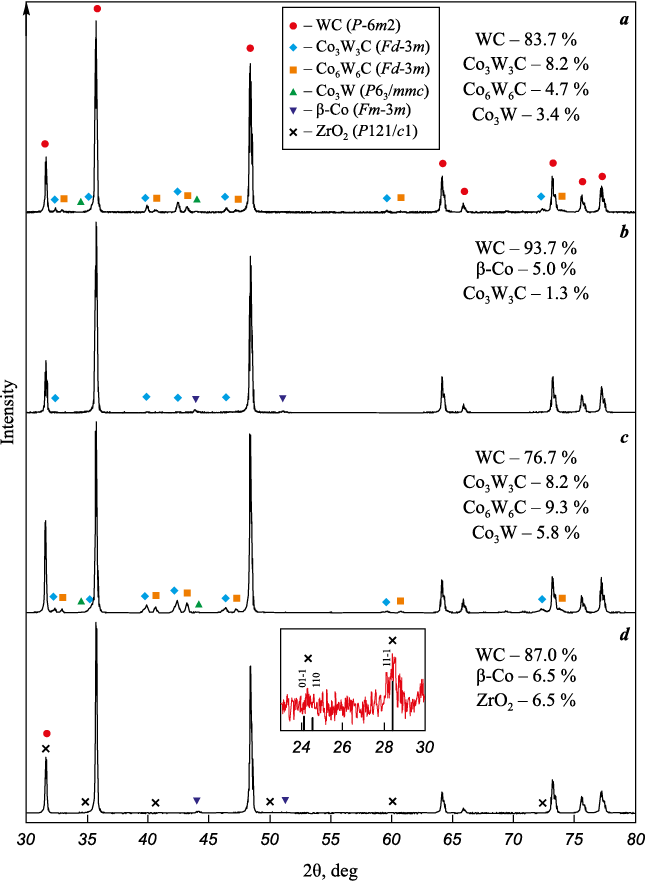
The carbon addition was calculated in a similar way, but by the change in the phase composition of the hard alloy made from the WC–6%Co nanocrystalline powder mixture. According to the phase composition of the sintered hard alloy (wt. %: 83.7 WC, 8.2 Co3W3C, 4.7 Co6W6C, 3.4 Co3W), its carbon content does not exceed 5.3 %, while it should be at least 5.8 %. Thus, the addition of carbon to the WC–6%Co powder mixture to compensate for its loss was 0.5 %.
Likewise, the addition of ZrC was also calculated from the change in the phase composition of the hard alloy, but in this case, the possible presence of oxygen on the surface of ZrC nanoparticles after milling was taken into account. Therefore, the case of a sufficient amount of oxygen on the surface of carbide particles to be removed after interaction with the carbide carbon mainly in the form of CO2 was considered, rather than in the form of CO, as was considered in the case with Al. A minimum of 1.3 % oxygen is required to bind 0.5 % carbon in CO2 . But to avoid the loss of carbon and completely bind this oxygen in ZrO2 , at least 4.2 % ZrC is required. Assuming that the interaction of impurity oxygen with carbide carbon can form not only CO2 , but also CO, 4.0 % of ZrC carbide was added to the WC–6%Co powder mixture before milling.
Nanocrystalline powder mixtures of WC–6%Co with and without additives were prepared using high-energy milling of microcrystalline WC powders (Dav ≈ 6 µm, Ctot = 6.15 %, Cfree = 0.07 %, Otot = 0.09 %, Kirovgrad hard alloys plant (KZTS), JSC, Kirovgrad), Co (Dav ≈ 3 µm, KZTS, JSC), Al (Dav ≈ 25 µm, RUSAL, Krasnoyarsk), ZrC (Dav ≈ 4 µm, Ctot = 10.26 %, Cfree = 1.72 %, Otot = 1.40 %, Donetsk Plant of Chemical Reagents, JSC (DZKhR), Donetsk) and carbon black (soot) grade T-900 (Dav ≈ 0.4 µm, Russia).
Milling of microcrystalline powders taken in a given ratio was carried out using a “Pulverisette 7” planetary ball mill (Fritsch, Germany) using grinding balls and grinding jar lining made of WC–6%Co hard alloy. The same grinding mode was used to prepare all powder mixtures: the rotation speed of the grinding jar support disk was 600 rpm; the weight of the powder taken for grinding was 10 g; the weight of the grinding balls with a diameter of 3 mm ~100 g; grinding jar volume – 45 mL; the volume of isopropyl alcohol C3H8O (high purity, 99.9 %, Component-Reaktiv, Ltd, Moscow) added during milling was 10 mL. After milling, the powder mixtures were dried in the vacuum drying cabinet VDL 23 (Binder, Germany) at a pressure of ~103 Pa and a temperature of 85 °C.
The compaction of powder mixtures was carried out at room temperature in a steel cylindrical mold with a punch diameter of 7.45 mm using uniaxial pressing at a pressure of ~460 MPa. Sintering of compact samples placed in graphite crucibles was carried out in a high-temperature vacuum furnace LF-22-2000 (Centorr/ Vacuum Industries, USA) for 15 min at t = 1380 °C in a vacuum of ~10–2 Pa. The heating rate to the temperature of sintering was 10 °C/min.
After sintering, the samples were cut in half along the cross section, the surface of which was then ground and polished on a “Buehler” machine (Germany) using grinding discs and diamond suspensions with a dispersion of 30 to 1 μm.
The crystal structure, phase composition, and lattice parameters of the powders were studied using X-ray diffraction on an XRD-7000 diffractometer (Shimadzu, Japan) with a Bragg–Brentano flat sample arrangement in the angle range 2θ from 10 to 140° with stepwise scanning ∆(2θ) = 0.03° and an exposure time of 2 s at a point and CuKα1.2 radiation. The X-ray phase analysis (XPA) of hard alloys was carried out on a STADI-P diffractometer (Stoe, Germany) with a Bragg–Brentano flat sample arrangement in the angle range 2θ from 5 to 120° with stepwise scanning ∆(2θ) = 0.03° and CuKα1.2 radiation. The X-ray patterns were analyzed by the Rietveld method using the X'Pert HighScore Plus Version 2.2e software package and the X-ray diffraction data library built into it. The broadening of diffraction reflections of WC was used to determine the average size of coherent scattering regions (DCSR) of X-rays and the magnitude of microstrains (ε).
Chemical analysis of powders for the content of total (Ctot ) and free (Cfree ) carbon was carried out using a “Metavak CS-30” analyzer (NPO Eksan, Izhevsk). The total oxygen content (Otot ) in these powders was determined by reductive melting in a carrier gas flow on an EMGA-620W/C gas analyzer (Horiba, Japan).
The morphology and particle size of powders, as well as the microstructure of hard alloys, were studied using a JSM 6390 LA scanning electron microscope (SEM) (Jeol, Japan) equipped with a JED 2300 analyzer (Jeol, Japan) for Energy Dispersive X-ray (EDX) analysis of the studied area.
The specific surface area (Ssp ) of the powders was measured by the Brunauer–Emmett–Teller (BET) adsorption method using a “Gemini VII” surface area and porosity analyzer (Micromeritics, USA) after degassing the powders in a vacuum of ~10 Pa at a temperature of 350 °C for 1 h. Assuming the approximation of the same size and spherical shape of particles, the average particle size \({D_{_{{\rm{BET}}}}} = \frac{6}{{{\rho _{{\rm{calc}}}}{S_{{\rm{sp}}}}}},\) was determined from the measured value Ssp , where ρcalc is the density calculated by the mixture rule according to the X-ray phase composition.
The density of hard alloys (ρmeas ) was determined using an “AccuPyc II 1340” helium pycnometer (Micromeritics, USA) and a measuring chamber with a volume of 1 cm3. The porosity of hard alloys was calculated according to the formula: \(p = \frac{{{\rho _{{\rm{calc}}}} - {\rho _{{\rm{meas}}}}}}{{{\rho _{{\rm{calc}}}}}} \cdot 100{\rm{ }}\% \).
The microhardness of hard alloys was measured according to the Vickers method on a MICROMET-1 microhardness tester (Buehler, Germany) with an automatic indentation of a diamond pyramid at a load of 200 g and a the duaration of loading of 10 s. At least 10 measurements (diamond pyramid indentations) were carried out on each sample, after which both diagonals were measured on each indentation, and the average microhardness value and the measurement error were determined from the data obtained.
Results and discussion
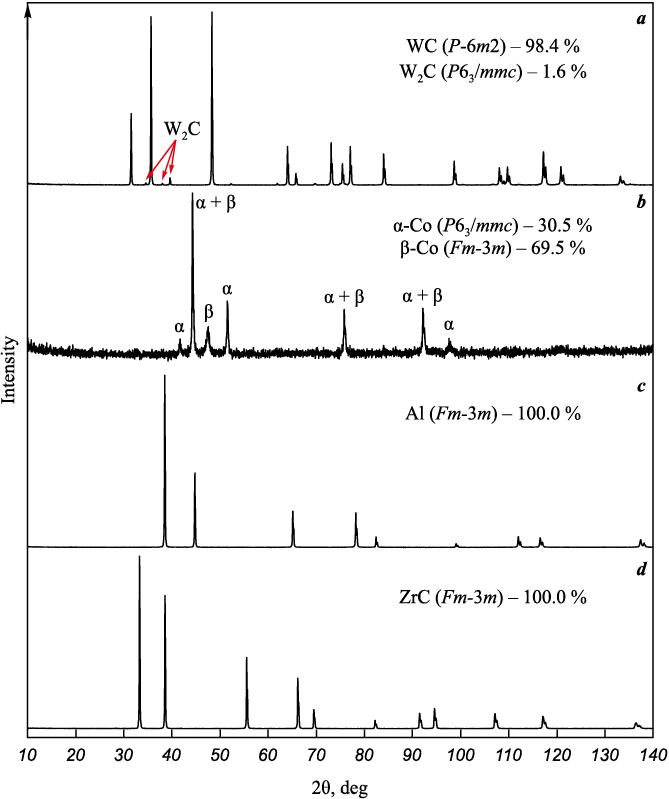
The X-ray diffraction patterns of all the initial powders (Fig. 1) used in this work for the preparation of nanocrystalline powder mixtures show rather narrow diffraction reflections, which confirms their coarseness. The WC powder is two-phase (Fig. 1, a) and, along with the main phase of hexagonal WC (sp. gr. P-6m2), contains a small amount of lower tungsten carbide W2C with a hexagonal structure (sp. gr. P63/ mmc), which indicates insufficient content of bound carbon in the W–C system. According to chemical analysis, the content of bound carbon (6.08 %) in WC powder is, indeed, lower than the stoichiometric value (6.13 %), however, there is present free carbon (0.07 %), therefore, the total carbon content in the powder (6.15 %) is sufficient to achieve single-phase WC during sintering. Cobalt Co powder (Fig. 1, b) is also two-phase and contains both crystalline modifications: low-temperature (up to 427 °C) α-Co with a hexagonal structure (sp. gr. P63/ mmc) and high-temperature (from 427 to 1495 °C) β-Co with a cubic structure (sp. gr. Fm-3m). Al (Fig. 1, c) and ZrC (Fig. 1, d) powders are single-phase and contain only cubic phases (sp. gr. Fm-3m) of Al and ZrC, respectively.
Fig. 1. X-ray diffraction patterns of the initial WC (a), Co (b), Al (c) and ZrC (d) powders |
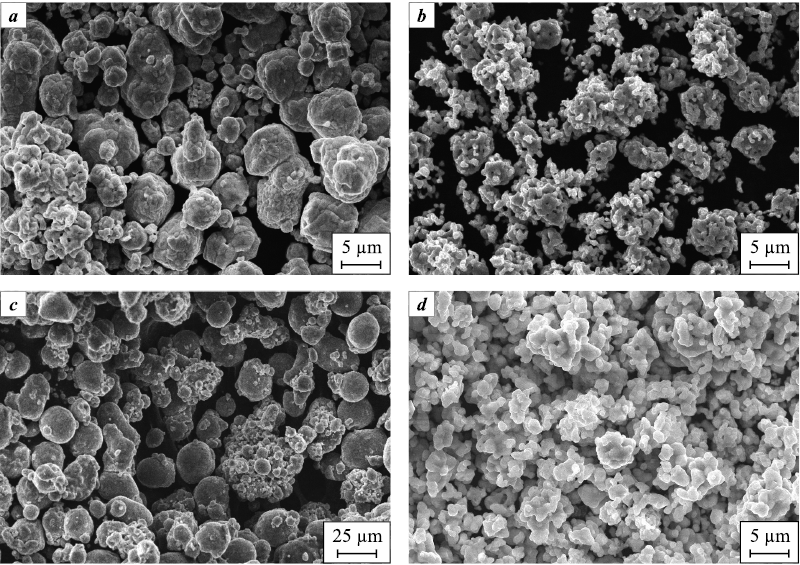
According to SEM images (Fig. 2), the Al powder (Fig. 2, c) contains the largest particles (up to 30–40 µm), which are several times larger than the particles of other powders. Co and ZrC powders, on the contrary, appear to be the most dispersed, showing very small rounded particles <1 µm in size, however, most of them are tightly bound together and form large agglomerates with a highly developed surface, ranging in size from hundreds of nanometers to several micrometers (Fig. 2, b, d). The WC powder (Fig. 2, a) is similar in particle morphology to Al powder (Fig. 2, c), but is closer to Co and ZrC powders in particle size and their agglomerates (Fig. 2, b, d).
Fig. 2. SEM images of the initial WC (a), Co (b), Al (c) and ZrC (d) powders |
Table 1 shows the average, maximum, and minimum particle sizes of the initial powders, determined from their SEM images, as well as their specific surface and the average particle size calculated from it.
Table 1. Characteristics of initial powders
|
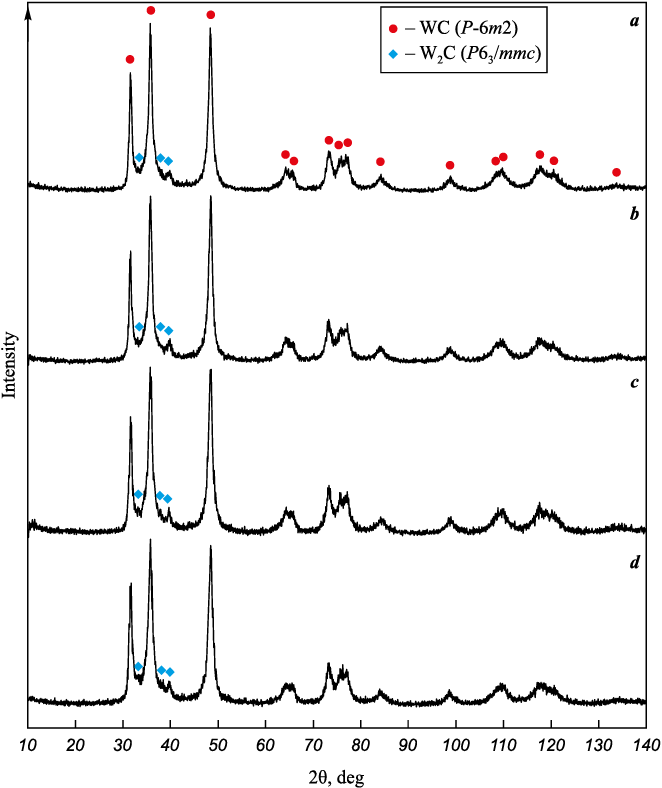
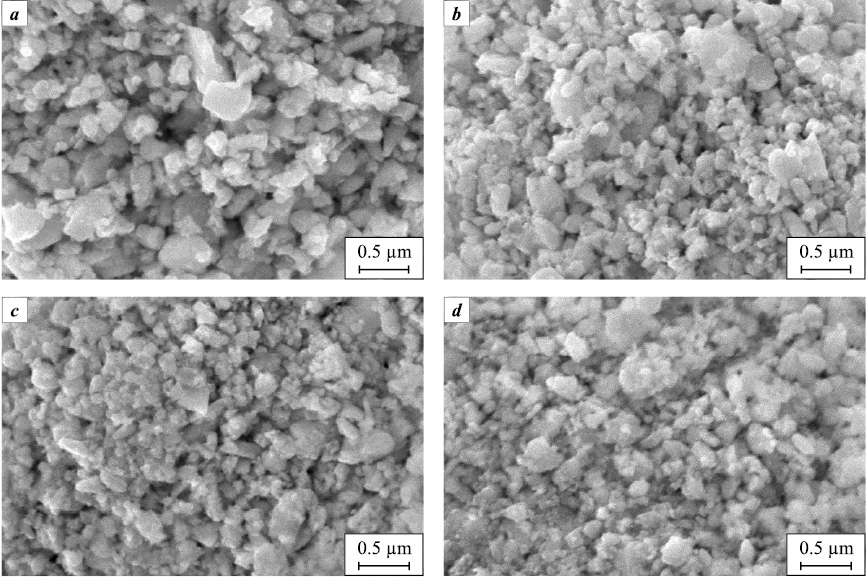
After preparation, all powder mixtures, according to X-ray diffraction (Fig. 3) and SEM (Fig. 4), look the same. The X-ray diffraction patterns of the mixtures (Fig. 3) show the same reflections as for the initial WC powder (see Fig. 1, a), which belong to the WC and W2C phases. However, due to the small size of the CSR and the presence of microdeformations, the diffraction reflections in the X-ray diffraction patterns of powder mixtures are noticeably broadened, due to which weak reflections of Co and Al or ZrC, are not visible. A quantitative analysis of the broadening of WC reflections showed that the average CSR sizes and microstrains for WC particles in all powder mixtures have close values (Table 2). The same is observed on the SEM images (Fig. 4), where powder mixtures have very little differences both in terms of particle size and morphology, although the initial powders were considerably different, especially Al powder (see Fig. 2).
Fig. 3. X-ray diffraction patterns of nanocrystalline powder mixtures
Fig. 4. SEM images of powder mixtures
Table 2. Characteristics of nanocrystalline powder mixtures
|
Thus, all the additives used in this work not only did not affect the grinding of WC, but themselves turned out to be grinded and subjected to grinding and uniform distribution throughout the volume of the powder mixture, including Al. This is also confirmed by the values of the specific surface area of powder mixtures (Table 2), which are an order of magnitude higher than the values for the initial powders (Table 1), whereas the average particle sizes in the mixtures calculated from the specific surface turned out to be close in magnitude to the average CSR sizes and do not exceed 100 nm (Table 2). It should be noted, however, that the additives introduced into the powder mixture, as expected, also introduced additional oxygen; its measured total content in nanocrystalline powder mixtures significantly exceeds the amount estimated based on the change in the phase composition (Table 2).
Despite the similarity of the obtained powder mixtures, hard alloys sintered from them significantly differ from each other both in phase composition (Fig. 5) and in microstructure (Fig. 6). On the X-ray diffraction pattern of the WC–6Co hard alloy (Fig. 5, a), which was sintered from the WC–6%Co powder mixture without any additives, diffraction reflections of three other phases are clearly visible in addition to the main phase WC, which indicate a carbon deficiency and are extremely undesirable in a hard alloy [41]. The addition of carbon to the powder mixture almost completely made up for its deficiency in the sintered hard alloy, its X-ray diffraction pattern of the main WC and Co phases are observed, however, weak lines of the Co3W3C η-phase are still present (Fig. 5, b). The addition of aluminum, on the contrary, only exacerbated the consequences of carbide decarburization, as a result of which the qualitative phase composition of the alloy became similar to the composition of the hard alloy from a powder mixture without additives (Fig. 5, a), while the content of undesirable phases increased (Fig. 5, c). Probably, during the preparation of the WC–6Co–0.4Al powder mixture, all the aluminium introduced was completely oxidized to Al2O3 during intensive grinding, and while sintering, instead of binding the oxygen adsorbed on the carbide particles, it, on the contrary, brought additional oxygen to its surface, resulting in an even greater loss of carbon. The addition of ZrC to the powder mixture allowed to preserve fully the WC and Co phases in the hard alloy sintered from the mixture, binding most of the adsorbed oxygen into the monoclinic ZrO2 oxide, as evidenced by the X-ray phase composition of the sintered alloy (Fig. 5, d).
Fig. 5. X-ray diffraction patterns of hard alloys |
According to the XRD results of hard alloys, the assessment of carbon loss (~0.5 %) and the amount of oxygen involved (~1.3 %) based on the change in the phase composition allowed for fairly accurate calculation of the amount of carbon and ZrC additives required to prevent WC decarburization. However, the measured total oxygen content in nanocrystalline powder mixtures (see Table 2) turned out to be several-fold higher than the estimated one. This means that only a part of the oxygen contained in the powder mixture is in the chemisorbed state, while the rest is present in other forms, including in the form of physically adsorbed water, which is removed upon heating without taking part in the decarburization of WC.
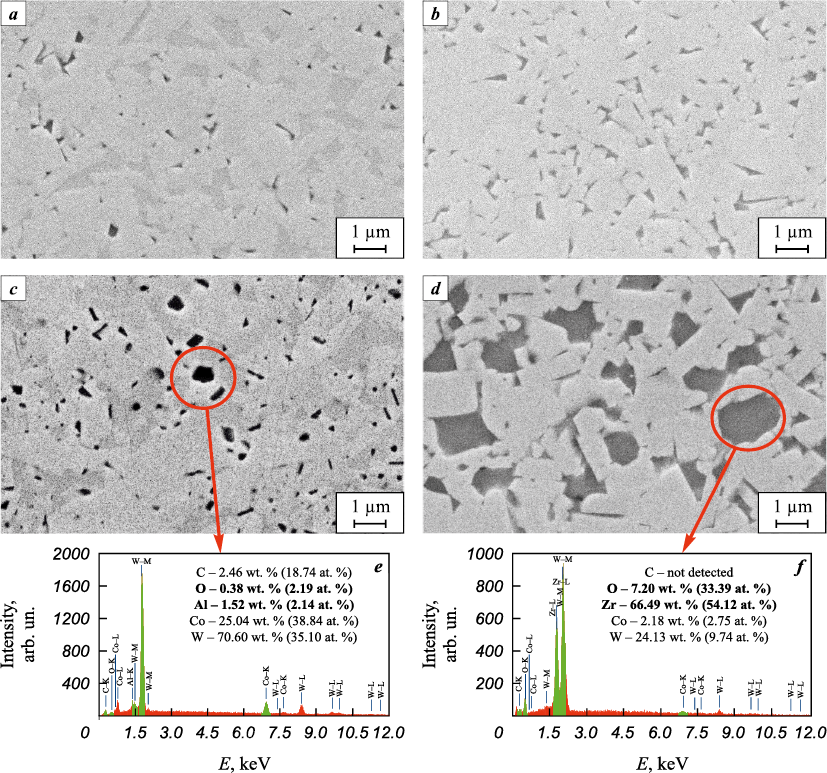
The microstructure of the WC–6Co alloy sintered from a powder mixture without additives (Fig. 6, a) looks rather dense and includes WC grains (light-colored), the space between which is filled with cobalt-containing phases (dark-colored) detected by X-ray diffraction (Fig. 5, a), and a few pores (black) no larger than 1 µm in size. Only WC grains and their intergrowths separated by a cobalt binder are observed in the microstructure of the WC–6Co–0.5C hard alloy sintered from a powder mixture with the addition of carbon (Fig. 6, b). As can be seen from XRD-analysis, the addition of Al to the powder mixture filled the microstructure of the hard alloy sintered from it (Fig. 6, c) with a large number of rounded inclusions (black-colored), resembling pores, among the grains of WC (light-colored) and cobalt-containing phases (dark-colored), (Fig. 5, c). The EDX analysis showed that the rounded dark areas observed in the microstructure of the WC–6Co–0.4Al hard alloy contain aluminum and oxygen (Fig. 6, c, e) and are Al2O3 particles and not pores. The authors of [36] reported similar inclusions in the WC–3Co–3Al2O3 alloy, detected using field emission scanning microscopy (FESEM) and EDX mapping (MAP), which showed that these are Al2O3 . The microstructure of the hard alloy (Fig. 6, d) made from a powder mixture with the addition of ZrC is a dense composition of carbide WC (light-colored) and oxide ZrO2 (dark-colored) grains surrounded by a cobalt layer, as confirmed by the EDX (Fig. 6, f ) and XFA (Fig. 5, d) analysis. Though in the WC–6Co–4ZrC powder mixture WC and ZrC nanoparticles are distributed uniformly over the entire volume, as it can be seen from the SEM images (Fig. 4, d), individual microcrystalline ZrO2 and WC grains formed in the microstructure of the hard alloy sintered from the powder, i.e. the growth of grains of various phases was not limited, as expected, on the contrary, it was supported.
Fig. 6. SEM images of hard alloys |
Table 3 shows the average, minimum, and maximum WC grain sizes determined from several SEM images for each hard alloy. According to these data, the carbon deficiency in the alloy inhibits the growth of WC grains, especially in the presence of Al2O3 particles, while the addition of carbon or ZrC, quite the contrary, promotes the growth of carbide grains, as confirmed by the average and maximum grain sizes in the hard alloy (Table 3). It is known that the presence of free carbon promotes the growth of WC grains during sintering, especially in the case of liquid-phase sintering [42]. According to [43; 44], during the oxidation of ZrC, there is a dissolution of oxygen in the carbide lattice; it is first accompanied by the formation of oxycarbide with the release of free carbon and zirconium from the carbide lattice; as oxygen further dissolves, the oxycarbide transforms into the cubic ZrO2 phase containing a certain amount of carbon, and then upon complete oxidation, it transforms into the ZrO2 monoclinic phase, which is seen in the X-ray diffraction pattern of the hard alloy (Fig. 5, d). Unlike ZrC, during the oxidation of WC, carbon leaves the carbide lattice in the form of CO/CO2 , leading to its decarburization [43; 45]. Thus, heating of the WC–6Co–4ZrC powder mixture, ZrC carbide not only binds the adsorbed oxygen into ZrO2 oxide, but also compensates for the loss of carbon in WC, as is the case with the addition of carbon in the WC–6Co–0.5C mixture.
Table 3. Characteristics of sintered hard alloys
| ||||||||||||||||||||||||||||||||||||||||||||||||
The additives used not only affected the microstructure of the hard alloy, but also led to a decrease in its density and microhardness (Table 3). The calculated density of the hard alloy with the WC–6Co phase composition is 14.97 g/cm3. However, due to the loss of carbon during sintering and the resulting formation of undesirable phases (Fig. 5, a), the density of the WC–6Co hard alloy (both calculated and measured) exceeded the expected one. The hard alloy WC–6Co–0.5C turned out to be closest to the WC–6Co hard alloy both in terms of phase composition and density. As X-ray diffraction patterns of hard alloys produced from powder mixtures WC–6Co–0.4Al and WC–6Co–4ZrC did not show reflections of the initial phases (Al or ZrC), while inclusions close in composition to Al2O3 and ZrO2 were found in the microstructure, the density of the samples ρcalc was calculated from the X-ray phase composition, taking into account the amount of oxide, into which the entire additive could turn. Calculations and measured density values of hard alloys showed that additions of carbon and ZrC, making up for the loss of carbon during sintering, contribute to the formation of a microstructure with the lowest porosity compared to carbon-deficient hard alloys (Table 3).
Besides, the deviation of the phase composition of the hard alloy from the perfect WC–6Co is accompanied by an increase in the inhomogeneity (scatter of values) of its microhardness, as confirmed by the measured value's error (Table 3). Overall, the measurements have shown that carbon deficiency leads to an increase in microhardness, while the presence of Al2O3 and ZrO2 oxide inclusions in the hard alloy microstructure, on the contrary, reduces it.
Conclusion
Homogeneous nanocrystalline powder mixtures with the average particle size not exceeding 100 nm based on WC–6Co with and without additives of C, Al, ZrC were prepared by high-energy milling from crystalline powders differing in their composition, properties, quantity, and average particle size. In the obtained mixtures, a high oxygen content was found; the amount of oxygen increases with the introduction of additives, especially Al. Though the original aluminum powder contained very large particles, it was completely oxidized during milling and turned into nanocrystalline oxide Al2O3 ; this fact only increased the loss of carbon during sintering and led to the formation of a multiphase and relatively porous microstructure of the hard alloy.
It was shown that only a part of oxygen contained in the powder mixtures is in the chemisorbed state and takes part in the decarburization of WC during vacuum sintering. The use of carbon and ZrC additives allowed to prevent the decarburization of WC during sintering of the hard alloy and to form a less porous microstructure in it. However, even the presence of ZrO2 inclusions could not impede the intensive growth of WC grains during sintering, on the contrary, it rather promoted it. Besides, the microhardness measurements have shown that carbon deficiency leads to an increase in microhardness, while the presence of Al2O3 and ZrO2 oxide inclusions in the hard alloy microstructure, on the contrary, reduces it.
References
1. Самойлов В.С., Эйхманс Э.Ф., Фальковский В.А., Локтев А.Д., Шкурин Ю.П. Металлообрабатывающий твердосплавный инструмент: Справочник. М.: Машиностроение, 1988. 368 с.
2. Фальковский В.А., Клячко Л.И. Твердые сплавы. М.: Изд. дом «Руда и металлы», 2005. 413 c.
3. Панов В.С., Чувилин А.М. Технология и свойства спеченных твердых сплавов и изделий из них. Учеб. пос. для вузов. М.: МИСИС, 2001. 432 c.
4. Lassner E., Schubert W.D. Tungsten compounds and their application. In: Tungsten: Properties, chemistry, technology of the element, alloys, and chemical compounds. Boston: Springer, MA, 1999. P. 133-177. https://doi.org/10.1007/978-1-4615-4907-9_4
5. Sarin V.K. Comprehensive hard materials. Oxford: Elsevier, 2014. 1806 p.
6. Gusev A.I., Kurlov A.S. Mechanical milling process modeling and making WC nanocrystalline powder. Inorganic Materials. 2009;45:35-42. https://doi.org/10.1134/S0020168509010063
7. El-Eskandarany M.S., Mahday A.A., Ahmed H.A., Amer A.H. Synthesis and characterizations of ball-milled nanocrystalline WC and nanocomposite WC-Co powders and subsequent consolidations. Journal of Alloys and Compounds. 2000;312(1-2):315-325. https://doi.org/10.1016/S0925-8388(00)01155-5
8. Isaeva N.V., Blagoveshchenskii Yu.V., Blagoveshchenskaya N.V., Mel'nik Yu.I., Samokhin A.V., Alekseev N.V., Astashov A.G. Preparation of nanopowders of carbides and hard-alloy mixtures applying low-temperature plasma. Russian Journal of Non-Ferrous Metals. 2014;55(6):585-591. https://doi.org/10.3103/S1067821214060108
9. McChandlish L.E., Seegopaul P. Development and applications of nanostructured tungsten carbide/cobalt powders. In: Proceedings of the European Conference on Advances in Hard Materials, Stockholm, 1996. P. 93-100.
10. Gao L., Kear B.H. Low temperature carburization of high surface area tungsten powders. Nanostructured Materials. 1995;5(5):555-569. https://doi.org/10.1016/0965-9773(95)00265-G
11. Fabijanic T.A., Alar Z., Coric D. Influence of consolidation process and sintering temperature on microstructure and mechanical properties of near nano- and nano-structured WC-Co cemented carbides. International Journal of Refractory Metals and Hard Materials. 2016;54:82-89. https://doi.org/10.1016/j.ijrmhm.2015.07.017
12. Breval E., Cheng J.P., Agrawal D.K., Gigl P., Dennis M., Roy R., Papworth A.J. Comparison between microwave and conventional sintering of WC/Co composites. Materials Science and Engineering: A. 2005;391(1-2): 285-295. https://doi.org/10.1016/j.msea.2004.08.085
13. Chuvil'deev V.N., Moskvicheva A.V., Boldin M.S., Sakharov N.V., Blagoveshchenskii Yu.V., Isaeva N.V., Mel'nik Yu.I., Shotin S.V., Nokhrin A.V. Spark plasma sintering of nanostructured tungsten carbide and carbide alloys. Vestnik of Lobachevsky University of Nizhni Novgorod. 2013;2(2):115-119. (In Russ.).
14. Kim H.C., Shon I.J., Jeong I.K., Ko I.Y., Yoon J.K., Doh J.M. Rapid sintering of ultra fine WC and WC-Co hard materials by high-frequency induction heated sintering and their mechanical properties. Metals and Materials International. 2007;13(1):39-45. https://doi.org/10.1007/BF03027821
15. Kelto C.A., Timm E.E., Pyzik A.J. Rapid omnidirectional compaction (ROC) of powder. Annual Review of Materials Science. 1989;19:527-550. https://doi.org/10.1146/annurev.ms.19.080189.002523
16. Raihanuzzaman R.M., Rosinski M., Xie Z., Ghomashchi R. Microstructure and mechanical properties and of pulse plasma compacted WC-Co. International Journal of Refractory Metals and Hard Materials. 2016;60:58-67. https://doi.org/10.1016/j.ijrmhm.2016.07.002
17. Wang X., Fang Z., Sohn H.Y. Nanocrystalline cemented tungsten carbide sintered by an ultra-high-pressure rapid hot consolidation process. In: Proceedings of the 2007 International Conference on Powder Metallurgy & Particulate Materials. Ed. J. Engquist. Denver, US, 2007;2:08-01.
18. Krokhalev A.V., Kharlamov V.O., Tupitsin M.A., Kuzmin S.V., Lysak V.I. Revisiting the possibility of formation of hard alloys from powder mixtures of carbides with metals by explosive compacting without sintering. Russian Journal of Non-Ferrous Metals. 2018;59(5):550-556. https://doi.org/10.3103/S1067821218050073
19. Raihanuzzaman R.M., Xie Z., Hong S.J., Ghomashchi R. Powder refinement, consolidation and mechanical properties of cemented carbides: An overview. Powder Technology. 2014;261:1-13. https://doi.org/10.1016/j.powtec.2014.04.024
20. Blagoveshchenskiy Y.V., Isayeva N.V., Blagoveshchenskaya N.V., Melnik Yu.I., Chuvildeyev V.N., Nokhrin A.V., Sakharov N.V., Boldin M.S., Smirnov Ye.S., Shotin S.V., Levinsky Yu.V., Voldman G.M. Methods of compacting nanostructured tungsten-cobalt alloys from nanopowders obtained by plasma chemical synthesis. Inorganic Materials: Applied Research. 2015;6(5):415-426. https://doi.org/10.1134/S2075113315050032
21. Norgren S., Garcia J., Blomqvist A., Yin L. Trends in the P/M hard metal industry. International Journal of Refractory Metals and Hard Materials. 2015;48:31-45. https://doi.org/10.1016/j.ijrmhm.2014.07.007
22. Garcia J., Cipres V.C., Blomqvist A., Kaplan B. Cemented carbide microstructures: A review. International Journal of Refractory Metals and Hard Materials. 2019;80:40-68. https://doi.org/10.1016/j.ijrmhm.2018.12.004
23. Fang Z.Z., Wang X., Ryu T., Hwang K.S., Sohn H.Y. Synthesis, sintering, and mechanical properties of nanocrystalline cemented tungsten carbide: A review. International Journal of Refractory Metals and Hard Materials. 2009;27: 288-299. https://doi.org/10.1016/j.ijrmhm.2008.07.011
24. Krasovskii P.V., Blagoveshchenskii Y.V., Grigorovich K.V. Determination of oxygen in W-C-Co nanopowders. Inorganic Materials. 2008;44(9):954-959. https://doi.org/10.1134/S0020168508090100
25. Kurlov A.S. Effects of vacuum annealing on the particle size and phase composition of nanocrystalline tungsten carbide powders. Russian Journal of Physical Chemistry A. 2013;87(4):654-661. https://doi.org/10.1134/S0036024413040158
26. Lantsev E.A., Malekhonova N.V., Tsvetkov Yu.V., Blagoveshchensky Yu.V., Chuvildeev V.N., Nokhrin A.V., Boldin M.S., Andreev P.V., Smetanina K.E., Isaeva N.V. Investigation of aspects of high-speed sintering of plasmachemical nanopowders of tungsten carbide with higher content of oxygen. Inorganic Materials: Applied Research. 2021;12(3):650-663. https://doi.org/10.1134/S2075113321030242
27. Kurlov A.S., Gusev A.I., Rempel' A.A. Vacuum sintering of WC-8wt.%Co hardmetals from WC powders with different dispersity. International Journal of Refractory Metals and Hard Materials. 2011;29(2):221-231. https://doi.org/10.1016/j.ijrmhm.2010.10.010
28. Kurlov A.S., Rempel' A.A., Blagoveshenskii Y.V., Samokhin A.V., Tsvetkov Yu.V. Hard alloys WC-Co (6 wt %) and WC-Co (10 wt %) based on nanocrystalline powders. Doklady Chemistry. 2011;439(1):213-218. https://doi.org/10.1134/S0012500811070068
29. Lantsev E.A., Malekhonova N.V., Nokhrin A.V., Chuvil'deev V.N., Boldin M.S., Andreev P.V., Smetanina K.E., Blagoveshchenskiy Yu.V., Isaeva N.V., Murashov A.A. Spark plasma sintering of fine-grained WC hard alloys with ultra-low cobalt content. Journal of Alloys and Compounds. 2021;857:157535. https://doi.org/10.1016/j.jallcom.2020.157535
30. Kurlov A.S., Yumasheva N.D., Danilov D.A. Concentration of oxygen and forms of it in TaC nanopowders. Russian Journal of Physical Chemistry A. 2019;93(3):501-508. https://doi.org/10.1134/S0036024419030117
31. Kurlov A.S., Yumasheva N.D., Danilov D.A. Vacuum annealing of TaC nanopowders. Russian Journal of Physical Chemistry A. 2020;94(7):1447-1455. https://doi.org/10.1134/S0036024420070183
32. Bokov A., Shelyug A., Kurlov A. Interplay between decarburization, oxide segregation, and densification during sintering of nanocrystalline TaC and NbC. Journal of the European Ceramic Society. 2021;41(12):5801-5812. https://doi.org/10.1016/j.jeurceramsoc.2021.05.007
33. Abyzov A.M. Aluminum oxide and alumina ceramics (Review). Pt. 1. Properties of Al2O3 and commercial production of dispersed Al2O3. Refractories and Industrial Ceramics. 2019;60(1):24-32. https://doi.org/10.1007/s11148-019-00304-2
34. Жигачев А.О., Головин Ю.И., Умрихин А.В., Коренков В.В., Тюрин А.И., Родаев В.В., Дьячек Т.А. керамические материалы на основе диоксида циркония. М.: Техносфера, 2018. 358 c.
35. Gordeev Yu.I., Abkaryan A.K., Zeer G.M. Design and investigation of hard metals and ceramics composites modified by nanoparticles. Perspektivnye materialy. 2012;(5):76-88. (In Russ.).
36. Fazili A., Nikzad L., RahimiPour M.R., Razavi M., Sala-hi E. Effect of Al2O3 ceramic binder on mechanical and microstructure properties of spark plasma sintered WC-Co cermets. International Journal of Refractory Metals and Hard Materials. 2017;69:189-195. https://doi.org/10.1016/j.ijrmhm.2017.08.010
37. Mukhopadhyay A., Chakravarty D., Basu B. Spark plasma-sintered WC-ZrO2-Co nanocomposites with high fracture toughness and strength. Journal of the American Ceramic Society. 2010;93(6):1754-1763. https://doi.org/10.1111/j.1551-2916.2010.03685.x
38. Xia X., Li X., Li J., Zheng D. Microstructure and characterization of WC-2.8wt% Al2O3-6.8wt% ZrO2 composites produced by spark plasma sintering. Ceramics International. 2016;42(12):14182-14188. https://doi.org/10.1016/j.ceramint.2016.06.044
39. Arenas F.J., Matos A., Cabezas M., Rauso C.D., Grigorescu C. Densification, mechanical properties and wear behavior of WC-VC-Co-Al hardmetals. International Journal of Refractory Metals and Hard Materials. 2001;19(4-6): 381-387. https://doi.org/10.1016/S0263-4368(01)00014-2
40. Andre B., Coulet M.-V., Esposito P.-H., Rufino B., Denoyel R. High-energy ball milling to enhance the reactivity of aluminum nanopowders. Materials Letters. 2013;110: 108-110. https://doi.org/10.1016/j.matlet.2013.07.101
41. Kurlov A.S., Gusev A.I. Tungsten carbides: Structure, properties and application in hardmetals. In: Tungsten carbides. (Springer series in materials science). Vol. 184. Springer, Cham., 2013. 242 p. https://doi.org/10.1007/978-3-319-00524-9
42. Konyashin I., Hlawatschek S., Ries B., Lachmann F., Dorn F., Sologubenko A., Weirich T. On the mechanism of WC coarsening in WC-Co hardmetals with various carbon contents. International Journal of Refractory Metals and Hard Materials. 2009;27(2):234-243. https://doi.org/10.1016/j.ijrmhm.2008.09.001
43. Войтович Р.Ф. Окисление карбидов и нитридов. Киев: Наук. думка, 1981. 192 с.
44. Gasparrini C., Chater R.J., Horlait D., Vandeperre L., Lee W.E. Zirconium carbide oxidation: Kinetics and oxygen diffusion through the intermediate layer. Journal of the American Ceramic Society. 2018;101(6):2638-2652. https://doi.org/10.1111/jace.15479
45. Kurlov A.S., Gusev A.I. Peculiarities of vacuum annealing of nanocrystalline WC powders. International Journal of Refractory Metals and Hard Materials. 2012;32:51-60. https://doi.org/10.1016/j.ijrmhm.2012.01.009
About the Authors
S. V. BriakunovRussian Federation
Sergey V. Briakunov - Engineer of the Laboratory of nonstoichio-metric compounds of Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences; Senior Lecturer of Electronic Engineering Department of Ural Federal University.
91 Pervomaiskaya Str., Ekaterinburg 620990; 19 Mira Str., Ekaterinburg 620002
A. S. Kurlov
Russian Federation
Aleksey S. Kurlov - Cand. Sci. (Phys.-Math.), Head of the Laboratory of nonstoichiometric compounds of Institute of Solid State Chemistry.
91 Pervomaiskaya Str., Ekaterinburg 620990
Review
For citations:
Briakunov S.V., Kurlov A.S. Microstructure and phase composition of hard alloys produced from nanocrystalline powder mixture WC-6wt.%Co with C, Al and ZrC additives. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(1):49-62. https://doi.org/10.17073/1997-308X-2023-1-49-62