Scroll to:
Investigation of physical, chemical, and technological properties of titanium powder obtained by thermal dehydrogenation in vacuum
https://doi.org/10.17073/1997-308X-2023-4-5-15
Abstract
In recent times, there has been significant interest in powder metallurgy, driven primarily by the active development of additive manufacturing. Consequently, a pressing task is the development of methods for producing initial metal powders that are cost-effective while meeting high consumer standards. This research is a continuation of studies on titanium powders obtained through SHS hydrogenation and thermal dehydrogenation. The titanium hydride powders, previously obtained using SHS technology, were sieved, resulting in fractions that matched the granulometric composition of titanium powders of PTK, PTS, PTM, and PTOM grades. Subsequently, the titanium hydride powder samples underwent dehydrogenation through vacuum annealing in an electric resistance furnace. Throughout the dehydrogenation process, the kinetics of hydrogen release from the titanium powder were examined as a function of particle size. The macro- and microstructure, chemical composition, and technological properties of the dehydrogenated powders were thoroughly analyzed. It was determined that the titanium powder maintained its original polygonal fragmented shape after dehydrogenation. The average particle size decreased by 5–20 %, and “satellites” were observed on larger particles. Chemical analysis revealed that larger samples contained a higher level of residual hydrogen and gas impurities (Σ 0.77 wt. %) compared to finer powders (Σ 0.26 wt. %). Regarding the study of technological properties, the resulting powders exhibited the necessary characteristics for use in titanium powder metallurgy, with the exception of low flowability due to the particle shape and microstructural heterogeneity). In conclusion, this research has demonstrated the potential of the SHS hydrogenation and thermal dehydrogenation method in producing high-quality titanium powders.
Keywords
For citations:
Cherezov N.P., Alymov M.I. Investigation of physical, chemical, and technological properties of titanium powder obtained by thermal dehydrogenation in vacuum. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(4):5-15. https://doi.org/10.17073/1997-308X-2023-4-5-15
Introduction
Titanium exhibits remarkably high chemical reactivity, in particular, it is highly reactive with nitrogen, carbon, and, most notably, oxygen, leading to the formation of a robust outer oxide layer [1]. This reactivity intensifies at elevated temperatures, leading to interactions with foundry equipment and the formation of a brittle surface layer on titanium, which can detrimentally affect its mechanical properties [2; 3]. Consequently, the production of titanium in conventional metallurgical furnaces becomes challenging, necessitating the creation of an inert gas or vacuum environment.
Powder metallurgy methods for crafting titanium products offer several advantages over traditional casting techniques:
– products and materials can attain unique physical, chemical, mechanical, and technological properties that are unattainable through conventional methods, such as pseudo-alloys, items with specific porosities, enhanced wear resistance, and friction materials;
– the possibility of using waste materials from metallurgical and engineering production, including scale and shavings;
– reduced consumption of costly metals due to the high material utilization ratio;
– less labor-intensive processes compared to casting and machining [4].
Titanium powder metallurgy involves stages like powder production, pressing, shaping, sintering, and finishing. The high cost associated with producing titanium powders often serves as the primary barrier hindering the utilization of powder metallurgy techniques for creating materials and products from titanium [5].
One potential approach to obtaining cost-effective titanium powders involves the crushing of titanium sponge [6; 7]. The resultant powder typically exhibits lower purity (96–97 wt. % Ti) and larger particle sizes (>630 µm). Challenges in the mechanical grinding process, using crushing methods, stem from the high ductility of pure titanium.
Electrolytic production of titanium is carried out at temperatures considerably below the titanium’s melting point, resulting in a cathode deposit comprised of crystalline dendritic intergrowths. These intergrowths disintegrate into individual particles during the cleaning process after removal from the electrolyte. This method of obtaining titanium from its dioxide eliminates several complex steps found in alternative methods, such as the production of titanium chlorides and the generation of a reducing agent. Consequently, the development of this method was of great interest. However, in all cases of electrolysis using an insoluble anode, the resulting titanium powder contains a notable amount of impurities, which is why these technologies have not gained widespread industrial adoption [8].
Titanium powders can also be produced by hydrogenating sponge or waste titanium to form titanium hydride, which possesses inherent brittleness. This titanium hydride can be easily mechanically crushed and sieved to yield fine powders, subsequently subjected to dehydrogenation (removing hydrogen in a vacuum) in a furnace to produce pure titanium powder [9; 10]. These powders exhibit an irregular and fragmented morphology, and their impurity content can be quite low provided that the impurity content of the initial feedstock is minimal. The key advantage of this method is its relatively low cost. The expenses associated with hydrogenation and dehydrogenation only marginally increase the cost of the starting material, and the resulting powder exhibits high purity when the impurity content of the raw material is controlled. The oxygen content is significantly influenced by the starting material, processing procedures, and the specific surface area of the powder [5].
High-purity titanium powders are typically manufactured using atomization methods, which involve melting the titanium material and atomizing the molten metal in an inert atmosphere through various techniques [11–14]. The atomization process yields spherical titanium powders, ideally suited for additive manufacturing. However, the disadvantages of these powders include a broad particle size distribution, ranging from a few micrometers to hundreds of micrometers, as well as their notably higher cost when compared to powders produced by other methods.
A thermochemical process used extensively in Russia for producing titanium powder involves the direct reduction of titanium oxide with calcium hydride. As the temperature gradually increases, calcium hydride dissociates into hydrogen gas and calcium. The liberated calcium subsequently reacts with titanium dioxide to form titanium metal and calcium oxide. Hydrogen gas released during the dissociation of calcium hydride partially interacts with the reduced titanium, leading to the formation of titanium hydride [14–16]. The resulting titanium powder, obtained through the reduction of titanium dioxide with calcium hydride, boasts a well-developed porous structure, low impurity content, and fine particle size.
The development of new production methods for titanium powder, such as the Armstrong, CSIRO, and MER processes, aims to reduce the cost of titanium powder. However, as of now, these methods have not found commercial applications [17].
Among the considered methods, hydrogenation-dehydrogenation technology is regarded as the most promising, as it allows for the production of low-impurity and cost-effective titanium powder [18; 19]. This technology is environmentally friendly and generates minimal waste. Moreover, it can utilize titanium waste materials like chips, sawdust, and small scraps as feedstock, making it resource-efficient [20; 21].
The efficiency of the hydrogenation-dehydrogenation technology can be further enhanced by employing the self-propagating high-temperature synthesis (SHS) method. SHS is unique in that the hydrogenation process occurs without the need for external energy input, relying solely on the heat generated by the exothermic reaction Ti + H2 → TiH2 + Q (39 kcal/mol) [22]. Subsequently, the synthesized titanium hydride undergoes the same processes as in the standard technology: grinding, sieving, and dehydrogenation.
The technological characteristics, such as bulk density and compressibility, of the initial powders are crucial for items produced through titanium powder metallurgy methods. These powders must possess specific properties and characteristics. The study of powder particle properties and structure for manufacturing products via solid-phase sintering is a pressing task in the development of titanium powder metallurgy technologies. It is essential for the quality parameters of powders to remain stable and consistent during storage [23–25].
The aim of this study was to conduct a thorough examination of titanium powders produced through the thermal dehydrogenation of titanium hydride in a vacuum. The research focused on investigating the impact of the powder’s particle size distribution on dehydrogenation parameters, structural characteristics, chemical properties, and technological indicators. The particle size distribution of the powders selected for this study was chosen with consideration for potential practical applications and aligned with the specifications of the PTK, PTS, PTM, and PTOM grades.
Experimental
Titanium hydride powders with varying particle size distributions were employed as initial materials. Each hydride sample matched the particle size distribution specifications of titanium powders corresponding to the PTK, PTS, PTM, and PTOM grades. All the titanium hydride powders were produced using the SHS hydrogenation technology described in [26].
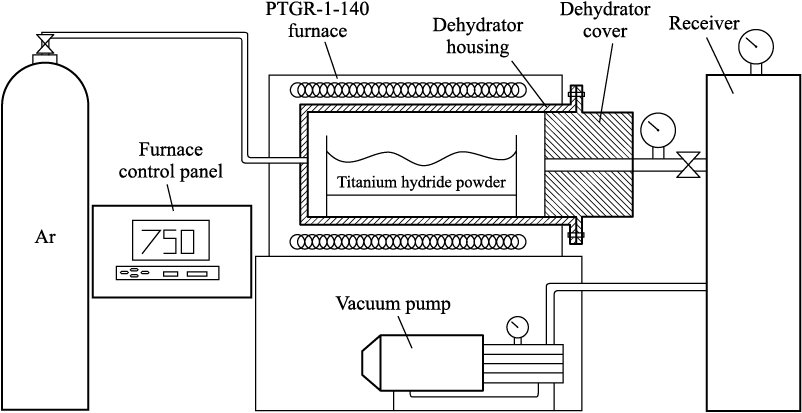
The thermal decomposition (dehydrogenation) of the titanium hydride powders was conducted using a specialized dehydrogenation apparatus, the schematic of which is depicted in Figure 1.
Fig. 1. Schematic view of facility for thermal dehydration |
The apparatus comprises a sealed cylindrical container constructed from stainless steel (dehydrator), which is situated inside the PTGR-1.0-140 electric furnace. Heating is regulated using a digital thermal controller, with a measurement error of ±5 °C. On both sides of the container, pipelines are connected for the supply of argon and connection to the vacuum pump.
For the dehydrogenation process, a 0.02 kg sample of titanium hydride powder was placed on a molybdenum substrate (boat). This boat was then positioned within the dehydrator on a designated stand. To create a vacuum, a 2NVR-5DM oil vane-rotor two-stage vacuum pump was utilized, capable of achieving a residual pressure of 2.6 Pa. To minimize the residual air content in the system, argon was introduced into the container at a pressure of 0.1 MPa, followed by evacuation. This procedure was carried out twice to ensure the lowest possible residual air content in the dehydrator.
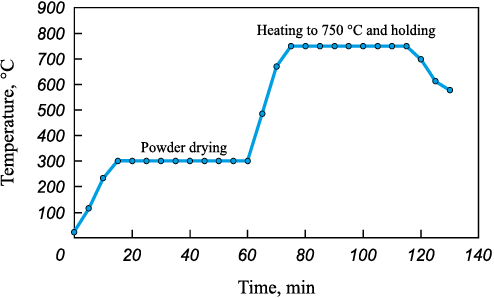
The dehydrogenation process (Fig. 2) entailed several steps. It commenced with vacuum drying at 300 °C for 60 min. Subsequently, the heating temperature was raised to 750 °C and held constant for 40 min. Thermal decomposition of the titanium hydride involved heating the powder until the pressure in the system ceased to change. The initiation and cessation of hydrogen release were determined by monitoring the vacuum gauge’s readings, indicating the start and end of pressure variations. The dehydrogenation parameters were chosen to prevent particle sintering. The cooling of the powders was carried out simultaneously with the furnace, and after complete cooling, the reactor was additionally purged with argon. The weight change of the dehydrogenated powder was calculated using equation:
| \[\Delta m = \frac{{{m_{\rm{h}}} - {m_{\rm{d}}}}}{{{m_{\rm{d}}}}} \cdot 100{\rm{ }}\% ,\] | (1) |
where mh is the weight of initial titanium hydride powder, and md is the weight of the powder after dehydrogenation. The measurement error for this calculation was ±0.1 g.
Fig. 2. Dehydration regime of powdered titanium samples |
The particle size of the obtained powders was determined using a “MicroSizer 201” laser particle analyzer (LLC “VA Insalt”, St. Petersburg, Russia). The measurement error did not exceed 1.2 %.
The particle morphology of the obtained titanium powder was examined using a “Zeiss Ultra plus” autoemission ultrahigh resolution scanning electron microscope (Carl Zeiss, Germany), which is based on “Ultra 55”. This microscope offers a magnification range of 12–106, an accelerating voltage of 0.02 V to 30 kV, and a probe current of 4–20 nA.
The chemical composition of the materials under investigation was determined using analytical chemistry methods: oxygen and nitrogen were analyzed through reductive melting in a graphite crucible in a helium current, carbon content was determined via oxidative melting in a ceramic crucible, and hydrogen content was determined according to State Standard GOST 24956–81. Oxygen and carbon were detected by measuring the amount of CO2 released through infrared absorption, while nitrogen was determined through thermal conductivity. The iron content in titanium was assessed using photocolorimetry. The instruments employed for these measurements include the TS-600 oxygen and nitrogen analyzer (Leco, USA), the RHEN-602 hydrogen analyzer (Leco, USA), the CS-600 carbon analyzer (Leco, USA), and the KFK-3-01 photometer (JSC “ZOMZ”, Zagorsk, Russia) for iron content determination.
The bulk density of the obtained powders was determined in accordance to State Standard GOST 19440–94, while their compressibility (compactibility) was assessed following State Standard GOST 25280–90. Pycnometric density was determined based on State Standard GOST 2211–2020, considering the weight of the analytical sample and its true volume, measured with a pycnometer using toluene as a saturating liquid.
The specific surface area was measured using low-temperature nitrogen adsorption with a “Sorbi-M” device designed for determining the specific surface of porous materials (ZAO “META”, Novosibirsk, Russia). Powder flowability was evaluated using a calibrated funnel (Hall device) in accordance with State Standard GOST 20899-98.
Result and discussions
Four distinct powder samples of dehydrogenated titanium, denoted as DH-PTK, DH-PTS, DH-PTM, and DH-PTOM, were obtained.
The initial titanium hydride powders contained 4.2 wt. % of hydrogen and primarily varied in their particle size distribution. When heated within the range of 300–400 °C, the process of titanium hydride decomposition initiates, leading to the release of hydrogen. However, even at higher temperatures, typically around 1000–1100 °C, dehydrogenation in this case doesn’t reach completion. To lower the temperature required for dehydrogenation, vacuum treatment is employed. Achieving the permissible hydrogen content for technical-grade titanium (<0.10 %) attained under vacuum conditions at t = 700÷800 °C. During the initial stages of dehydrogenation, when the hydrogen content in titanium is relatively high, the rate of hydrogen release is notably significant [27].
During dehydrogenation, it was observed that the particle size plays a role in influencing the kinetics of hydrogen release (Table 1). The PTOM sample, characterized by the smallest particle size, exhibits the commencement of hydrogen desorption at a low temperature, tin = 520 °C. For the coarser PTS and PTM powders, hydrogen release initiates at 550 °C and 540 °C, respectively. It can be inferred that larger particles require more heat to initiate this process, as evident in the case of the PTK sample, where hydrogen release commences at tin = 555 °C.
Table 1. Kinetics of dehydration of powdered samples of titanium hydride
| ||||||||||||||||||||||||||||||
Moreover, for the powders under examination, the time interval from the initiation of hydrogen release to its completion also differs. As particle size decreases, this time interval tends to increase, possibly due to incomplete dehydrogenation of the coarser particles.
To evaluate the extent of dehydrogenation, the samples were weighed both before and after the dehydrogenation process. It’s important to note that as the particle size of the samples decreases, the reduction in weight after dehydrogenation becomes more significant. As indicated in Table 1, it is evident that coarse particles are not completely dehydrogenated, which is reflected in the short duration of hydrogen release and the relatively low weight loss.
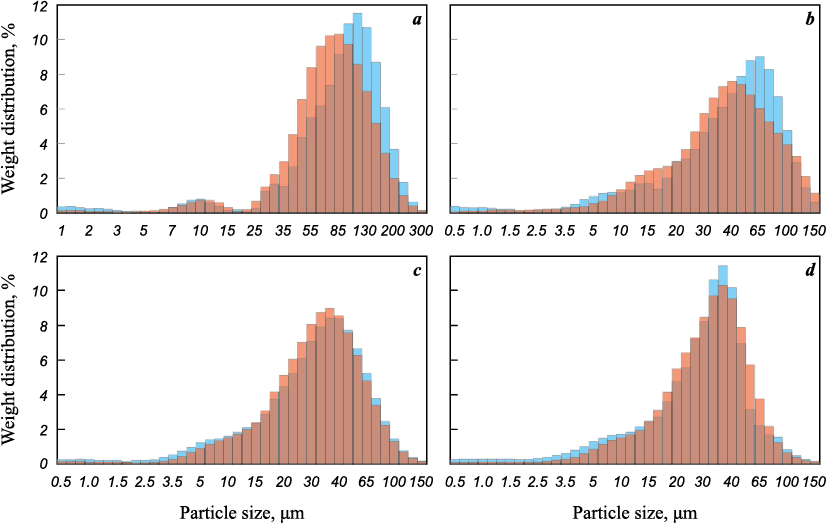
The histograms in Figure 3 reveal that following the thermal decomposition of titanium hydride, there is a slight reduction in the particle size within the overall mass. This phenomenon is similar to what was observed in a previous study [28], where it was demonstrated that the thermal decomposition of scandium hydride powder results in a marginal decrease in the average linear particle size (by ~4 %). As reported in other publications [29], during the hydrogenation of titanium, there is an expansion of the unit cell volume by approximately 2.5 times, leading to a “swelling” of the particles. Presumably, after the removal of hydrogen, the titanium particles tend to revert to their original structure under the influence of temperature, causing them to decrease in volume. This effect is more pronounced in the PTK and PTS powder samples, which consist of coarser particles when compared to PTM and PTOM. The average linear particle size after dehydrogenation for the PTK, PTS, PTM, and PTOM samples decreased by roughly 24, 13, 12 and 10 %, respectively. Additionally, after dehydrogenation, there was a reduction in the number of particles within the range of 0.5 to 10 μm. It is likely that, at the chosen temperature, such particles are sintered with the primary fraction particles.
Fig. 3. Histograms of particle size distribution of powdered titanium before ( |
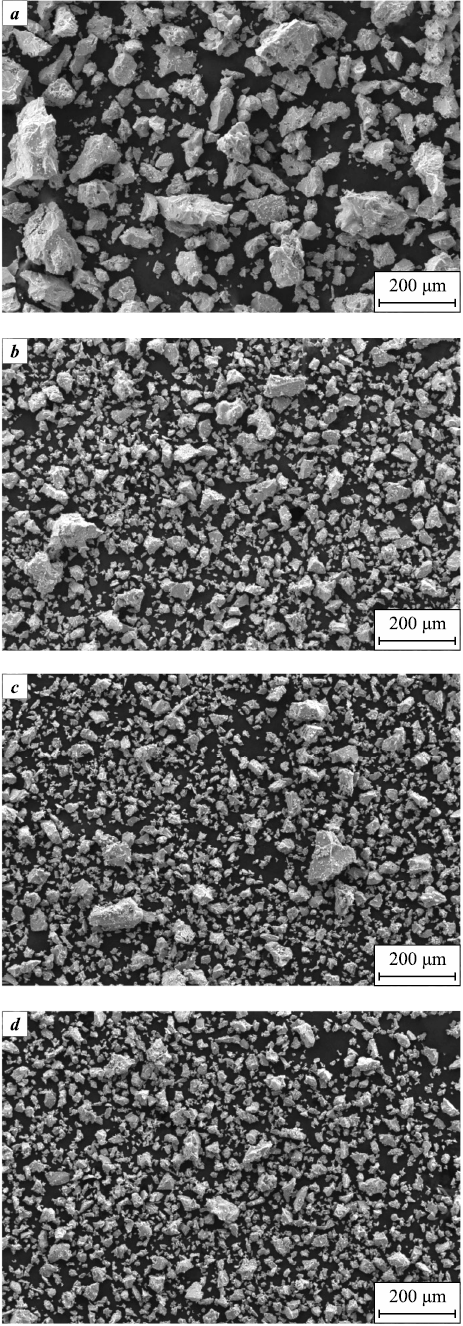
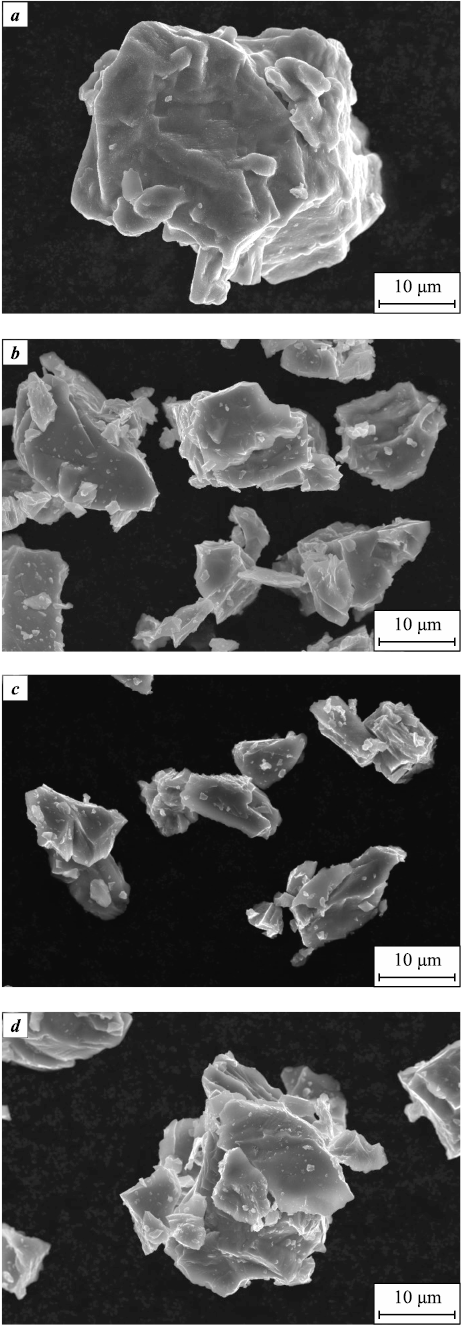
The structure of particles has a significant impact on the properties of titanium powder, and it is influenced by the methods used for production and powder processing, as well as the chemical composition. Electron microscopy was employed to examine the dehydrogenated titanium powder, and the results indicated that the particle shape of the original titanium hydride was preserved. Figure 4 provides an overall view of the dehydrogenated titanium powders obtained. It is noteworthy that the chosen dehydrogenation temperature did not alter the shape of the powder particles, which retained their polygonal fragmented structure.
Fig. 4. General view of dehydrogenated titanium powders |
In the detailed images of Figure 5 at higher magnification, individual particles of the dehydrogenated titanium powder can be closely examined. A portion of the fine particles tends to sinter with the coarser particles, giving rise to what are commonly referred to as “satellites.” These satellite formations result in various types of micro-irregularities in the structure, which can potentially have adverse effects on the technological properties of the powders and the properties of the final powder products. The formation of satellites is influenced by the vacuum treatment and the specific temperature applied during dehydrogenation. As lowering the temperature and reducing the vacuum level are generally undesirable, it is recommended to pre-sieve the powder before dehydrogenation to eliminate fractions within the 0.5 to 10 μm range. It is worth noting that the surface of the titanium particles after dehydrogenation is characterized by the absence of pores and does not exhibit a developed surface structure.
Fig. 5. Microstructure of dehydrogenated titanium powders |
The purity of titanium can be estimated through its hardness (HB), which tends to increase with higher impurity content. Among the impurities, oxygen and nitrogen, which form interstitial solid solutions with titanium, have a significant detrimental impact on the ductility of titanium and are considered harmful. The primary impurities also include carbon and iron. The influence of these main impurity elements on the hardness of titanium can be roughly expressed by the following empirical equation, MPa [27]:
| \[HB = 1960\sqrt {{\rm{N}}{\rm{, \% }}} + 1580\sqrt {{\rm{O}}{\rm{, \% }}} + 450\sqrt {{\rm{C}}{\rm{, \% }}} + 200\sqrt {{\rm{Fe}}{\rm{, \% }}} + 57.\] | (2) |
As per Equation (2), nitrogen has the most substantial effect on the hardness of titanium, followed by oxygen, carbon, and iron. Nitrogen, which stabilizes the α phase of titanium, raises the temperature of the polymorphic transformation. Every 0.01 % increase in nitrogen content results in a 20 MPa increase in tensile strength and a 60 MPa increase in hardness. Oxygen also stabilizes the α phase, and a 0.1 % increase in oxygen content leads to a 12 MPa increase in tensile strength and a 40 MPa increase in hardness. Nitrogen and oxygen are interstitial atoms with high solubility in α titanium and are situated in octahedral voids, which enhances the rigidity of the interatomic bonds in titanium. Hydrogen is another highly detrimental impurity in titanium, as it significantly diminishes the ductility of the metal, particularly its impact toughness. Carbon has limited influence on the mentioned properties of titanium, as it only dissolves slightly in α titanium. The presence of iron impurities up to 0.5 % has a negligible impact on the mechanical properties of titanium.
The quality of products manufactured from titanium powders is directly linked to the purity of the initial powders. Thus, it is crucial to maintain the concentration of major impurities at a minimum level. Based on the results of chemical analysis (Table 2) of dehydrogenated titanium powders, it was observed that samples from the coarser fraction contain a higher level of residual hydrogen (0.2 wt. %) compared to the finer fraction (0.06 wt. %). This suggests that the selected dehydrogenation process is less effective for the PTK and PTS samples. Notably, during the dehydrogenation process, there is a reduction in gas impurities such as nitrogen and oxygen. This may be due to the vacuum facilitating active degassing of the powders [30]. Additionally, the hydrogen released during dehydrogenation can serve as a reducing agent, forming molecules with nitrogen and oxygen impurities, which are subsequently desorbed into the gas phase and eliminated [31; 32]. As a result, the PTM and PTOM powder samples exhibit the lowest levels of gas impurities (0.5–0.6 wt. %).
Table 2. Content of major impurities in the studied powder samples
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
For practical applications, the technical characteristics of titanium powders are of great importance. The size and shape of particles are the primary factors that determine the technological properties of powders, including bulk density, flowability, and compressibility. The technological properties of dehydrogenated titanium powder are summarized in Table 3.
Table 3. Technological properties of the studied powdered samples
|
Bulk density represents a volumetric property of the powder, which is the ratio of its weight to its volume at free bulk density. The bulk density of dehydrogenated titanium powder increases in comparison to the hydride due to the higher density of pure titanium (4.5 g/cm3). Additionally, there is a trend of decreasing bulk density with a reduction in the average particle size. Fine particles possess a higher specific surface area, which increases inter-particle friction, making it more challenging for them to move relative to each other, resulting in a lower bulk density.
Compactibility characterizes the change in powder density during compression and determines the ease and cost-effectiveness of the cold pressing process. Powder samples with larger particle sizes exhibit lower compactibility compared to titanium hydride, which, due to its brittleness, breaks down and fills voids. Pure titanium powder is more pliable, and therefore compacting the coarse fraction at a pressure of 200 MPa is challenging. The compactibility of dehydrogenated powder samples, in general, aligns with that of the initial titanium hydride powders.
The pycnometric (true) density of metal powders depends on their internal porosity, defects in the crystal lattice, oxide content, and usually differs from the theoretical density. Based on measurements of the pycnometric density of the dehydrogenated powder samples, it is evident that the PTK powder sample, with the highest impurity content (particularly hydrogen), has a lower density (4.43 g/cm3). Generally, there is a dependency: the closer the pycnometric density is to the theoretical density, the lower the impurity content in the samples.
In evaluating the technological properties of powder materials, the specific surface area of their particles is significant in several instances. Its value can provide insights into certain physical and chemical properties of powder materials, including the degree of dispersibility. As the average particle size of the powder samples decreases during dehydrogenation and the particle area increases due to satellites, the specific surface area of the dehydrogenated powders (0.7–0.9 m2/g) is greater compared to that of the hydride (0.6–0.7 m2/g).
Flowability denotes the ability of powders to flow out from the opening of a container under the influence of gravity. Flowability is influenced by factors such as powder density, particle size and shape, surface condition, humidity, and the nature of contact between particles. Good flowability is particularly important in cases of automatic pressing, where the productivity of the press is dependent on the rate of mold cavity filling. Poor fluidity can also lead to the production of items with non-uniform density. The resulting powders, due to the fragmented shape of the particles and structural defects, exhibit a low level of flowability, amounting to 16.6 s for the coarse fraction, while the fine fraction does not flow.
Conclusions
The presented study examines the impact of the particle size distribution of initial titanium hydride powders on their thermal decomposition process. It comprehensively investigates the physical, chemical, and technological properties of dehydrogenated titanium powders.
It has been determined that the chosen dehydrogenation process is better suited for the fine fraction with an average particle size of 35 µm, where complete dehydrogenation occurs without particle sintering. Larger particles (>60 µm) require higher dehydrogenation temperatures. During the thermal decomposition of titanium hydride, there is a significant reduction in the average linear size of powder particles, ranging from 5 to 20 % depending on the sample.
The dehydrogenation process does not alter the shape of the particles, which maintain their original polygonal fragmented structure. The study identifies the presence of “satellites” on large particles, which can introduce various forms of micro-irregularities in the structure, potentially affecting the technological properties of the powders and the properties of the final powder products. This factor should be taken into consideration during production, and powder separation should be carried out with care.
Chemical analysis results reveal that larger powder samples contain a higher amount of gas impurities (hydrogen, nitrogen, oxygen Σ 0.77 wt. %) compared to the fine fraction (Σ 0.26 wt. %). Presumably, this is due to the more complete dehydrogenation and degassing of the fine fraction under the selected regime.
The technological properties of dehydrogenated powders generally meet the requirements for their use in powder metallurgy. However, the presence of satellites and the fragmented shape of the particles significantly reduce the fluidity of the powders, which can lead to difficulties when using them in automated processes.
In conclusion, this comprehensive study of titanium powders obtained through SHS hydrogenation and thermal dehydrogenation demonstrates their potential for application in powder metallurgy.
References
1. Mal’shin V.M., Zavadovskaya V.N., Pampushko N.A. Metallurgy of titanium. Moscow: Metallurgiya, 1991. 208 p. (In Russ.).
2. Bolzoni L., Ruiz-Navas E.M., Gordo E. Powder metallurgy CP–Ti performances: Hydride–dehydride vs. sponge. Materials & Design. 2014;60:226–232. https://doi.org/10.1016/j.matdes.2014.04.005
3. Gramata V.A., Petrun’ko A.N., Galickii N.V., Olesov Yu.G., Sandler R.A. Titan. Moscow: Metallurgiya, 1983. 539 p. (In Russ.).
4. Panda A., Dobránsky J., Jančík M. Pandová I., Kačalová M. Advantages and effectiveness of the powder metallurgy in manufacturing technologies. Metalurgiya. 2018;57(4):353–356.
5. Fang Z.Z., Paramore J.D., Sun, P., Ravi Chandran K.S., Zhang Y., Xia Y., Cao F., Koopman M., Free M. Powder metallurgy of titanium – past, present, and future. International Materials Reviews. 2017;63(7):1–53. https://doi.org/10.1080/09506608.2017.1366003
6. Antsiferov V.N., Smetkin A.A., Yarmonov A.N., Peshcherenko S.N. Method for producing titanium powder: Patent 2178341 (RF). 2002. (In Russ.).
7. Rymkevich D.A., Tankeev A.B., Bezdolja I.N., Teterin V.V., Permjakov A.A. Method of obtaining sponge titanium: Patent 2466198 (RF). 2015. (In Russ.).
8. Withers J.C. 3 – Production of titanium powder by an electrolytic method and compaction of the powder. In book: Titanium powder metallurgy. 2015. P. 33–49. https://doi.org/10.1016/B978-0-12-800054-0.00003-4
9. Yanko T., Brener V., Ovchinnikov O. Production of spherical titanium alloy powders used in additive manufacturing from titanium scrap. In: MATEC Web of Conferences (The 14th World Conference on Titanium (Ti 2019), Nantes, France, 10–14 June 2019). 2020;321(07008). https://doi.org/10.1051/matecconf/202032107008
10. Goso X.C., Kale A. Production of titanium metal powder by the HDH process. Journal of the Southern African Institute of Mining and Metallurgy. 2011;111(3):203–210.
11. Heidloff A., Rieken J., Anderson I., Byrd D., Sears J., Glynn M., Ward R. Advanced gas atomization processing for Ti and Ti alloy powder manufacturing. The Journal of the Minerals, Metals & Materials Society (JOM). 2010;62:35–41. https://doi.org/10.1007/s11837-010-0075-x
12. Alishin M.I., Knjazev A.E. Production of metal powder compositions of high purity titanium alloys by induction gas atomization for additive technologies. Trudy VIAM. 2017;(11):35–43. (In Russ.). https://dx.doi.org/ 10.18577/2307-6046-2017-0-11-5-5
13. Novikov A.N. A method for producing ultrafine powders of metal or metal alloys: Patent 2588931 (RF). 2016. (In Russ.).
14. Sun P., Fang Z.Z., Xia Y., Zhang Y., Zhou C. A novel method for production of spherical Ti–6Al–4V powder for additive manufacturing. Powder Technology. 2016;301: 331–335. https://doi.org/10.1016/j.powtec.2016.06.022
15. Kasimtsev A.V., Zhigunov V.V., Tabachkova N.Y. The composition, structure, and properties of calcium-hydride powder of titanium carbide. Russian Journal of Non-Ferrous Metals. 2009;50(3):276–280. https://doi.org/10.3103/S1067821209030171
16. Xia Y., Fang Z.Z., Zhang T., Zhang Y., Sun P., Huang Z. Deoxygenation of titanium hydride with calcium hydride. In: Proceedings of the 13th World Conference on Titanium Ti-2015 (Manchester Grand Hyatt, San Diego, California, USA, 16–20 August 2015). John Wiley & Sons, Inc., 2016. P. 135–137. https://doi.org/10.1002/9781119296126.ch20
17. Neikov O.D., Gopienko V.G. Production of titanium and titanium alloy powders. In: Handbook of non-ferrous metal powders. Elsevier Ltd., 2009. P. 549–570. https://doi.org/10.1016/B978-0-08-100543-9.00018-X
18. Ivasishin O., Moxson V. 8 – Low-cost titanium hydride powder metallurgy. In: Titanium powder metallurgy. Elsevier Inc., 2015. P. 117–148. https://doi.org/10.1016/B978-0-12-800054-0.00008-3
19. Abakumov G., Duz V., Ivasishin O., Moxson V., Savvakin D. High performance titanium powder metallurgy components produced from hydrogenated titanium powder by low cost blended elemental approach. In: Proceedings of the 12th World Conference on Titanium Ti-2011 (Beijing, China, 19–24 June 2011). Beijing: Science Press, 2012. Vol. 2. P. 1639–1643.
20. Beshkarev V.T., Gasanov A.A., Yuzhakova E.A., Ivanov V.V., Kartsev V.E., Kotlyarov V.I., Kozlov R.Yu. Method for obtaining finely dispersed spherical titanium-containing powders: Patent 2631692 (RF). 2016. (In Russ.).
21. Oh J.M., Roh K.M., Lee B.K., Suh C.Y., Kim W., Kwon H., Lim J.W. Preparation of low oxygen content alloy powder from Ti binary alloy scrap by hydrogenation–dehydrogenation and deoxidation process. Journal of Alloys and Compounds. 2014;593:61–66. https://doi.org/10.1016/j.jallcom.2014.01.033
22. Doluhanjan S.K. SHS is a method for producing hydrogen storages. Al’ternativnaya energetika i ekologiya. 2005;(11):13–16. (In Russ.).
23. Pavlenko D.V. Influence of titanium powder parameters on the strength of sintered semi-finished products. Novі materіali і tekhnologіi v metalurgіi ta mashinobuduvannі. 2014;(2):87–92. (In Russ.).
24. Knyazev A.E., Nerush S.V., Alishin M.I., Kuko I.S. Investigations of technological properties of metal powder compositions of titanium alloys VT6 and VT20 obtained by induction melting and gas atomization. Trudy VIAM. 2017;59(11):44–53. (In Russ.). https://doi.org/10.18577/2307-6046-2017-0-11-6-6
25. Chen B., Shen J., Ye X., Umeda J., Kondoh K. Advanced mechanical properties of powder metallurgy commercially pure titanium with a high oxygen concentration. Journal of Materials Research. 2017;32(19):3769–3776. https://doi.org/10.1557/jmr.2017.338
26. Cherezov N.P., Alymov M.I. Structure and properties of titanium hydride powder obtained from titanium sponge by SHS hydrogenation. Powder Matallurgy and Functional Coatings. 2022;16(4):15–24. (In Russ.). https://dx.doi.org/10.17073/1997-308X-2022-4-15-24
27. Ustinov V.S., Olesov Yu.G., Antipin L.N., Drozdenko V.A. Titanium powder metallurgy. Moscow: Metallurgiya, 1973. 248 p. (In Russ.).
28. Tsarev M.V., Zabavin E.V., Mokrushin V.V., Berezhko P.G. Change in the particle size of powdered metals during their hydrogenation and dehydrogenation. In: Interaction of hydrogen isotopes with structural materials. IHISM’11 JUNIOR: The Seventh International School of young scientists and specialists named after A.A. Kurdyumov (Zvenigorod, 24–28 October 2011). Sarov: FGUP ״RFYaTs–VNIIEF״, 2012. P. 379–384. (In Russ.).
29. Rudskih V.V., Volkova T.S., Levchenkova O.N., Zharkov A.Yu., Svetlakov S.V. Hydrogenation of titanium fractions at reduced hydrogen pressure. In: Interaction of hydrogen isotopes with structural materials. IHISM’14: The Fifth International Conference and the Ninth International School of young scientists and specialists named after A.A. Kurdyumov (Sarov, 7–11 June 2014). Sarov: FGUP ״RFYaTs–VNIIEF״, 2014. P. 265–276. (In Russ.).
30. Shapovalova O.M., Babenko E.P. The influence of the heating temperature on the process of gas saturation of titanium powders. Novі materіali і tekhnologіi v metalurgіi ta mashinobuduvannі. 2008;(2):93–98. (In Russ.).
31. Liu H., Lixian L., Liu Y. Vacuum activation assisted hydrogenation-dehydrogenation for preparing high-quality zirconium powder. Materials and Manufacturing Processes. 2019;34(6):630–636. https://dx.doi.org/ 10.1080/10426914.2019.1566610
32. Park Choi, Kang Jang-Won. Oxygen reduction behavior of HDH TiH2 powder during dehydrogenation reaction. Metals. 2019;9(11):1154. https://doi.org/10.3390/met9111154
About the Authors
N. P. CherezovRussian Federation
Nikita P. Cherezov – Junior Researcher of the Laboratory of high-energy methods of synthesis of ultrahigh-temperature ceramic materials
8 Akademican Osip'yan Str., Chernogolovka, Moscow Region 142432, Russia
M. I. Alymov
Russian Federation
Mikhail I. Alymov – Dr. Sci. (Eng.), Corresponding Member of the Russian Academy of Sciences, Director
8 Akademican Osip'yan Str., Chernogolovka, Moscow Region 142432, Russia
Review
For citations:
Cherezov N.P., Alymov M.I. Investigation of physical, chemical, and technological properties of titanium powder obtained by thermal dehydrogenation in vacuum. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(4):5-15. https://doi.org/10.17073/1997-308X-2023-4-5-15
JATS XML