Scroll to:
Investigation of the properties of high-strength fibers by methods of physico-chemical analysis
https://doi.org/10.17073/1997-308X-2023-4-34-40
Abstract
The carbon fiber (CF) of UMT 49-12K-ER grade, manufactured by Alabuga-Volokno LLC (Umatex JSC), was the subject of an extensive study. This investigation encompassed an analysis of its physico-chemical properties. The interplanar dimensions and chemical composition of the CF were determined using X-ray diffraction and atomic emission spectroscopy. Surface properties of the CF, including specific surface area and pore size distribution, were investigated through nitrogen adsorption. The BET specific surface area was measured at 0.29 m2/g. The volume of mesopores and their size distribution were calculated using the Barrett, Joyner, and Halenda method. Additionally, an analysis of surface functional groups was conducted through a back titration method. It was observed that there was no presence of carboxyl, phenolic, or carbonyl groups. The diffraction patterns were processed with a two-component profile description model. The results of atomic emission spectral analysis revealed that silicon compounds were the dominant impurities in the chemical composition of the CF. Further investigations determined that, in an inert environment, the epoxy coupling agent used to enhance the performance properties of this CF undergoes thermal decomposition at temperatures of 300–400 °C. The CF itself does not experience weight loss when heated up to 950 °C. It was also discovered that this CF ignites in the presence of oxygen at temperatures exceeding 550 °C, surpassing the thresholds noted in previous publications for carbon fibers without such specialized additives. The results of this research have suggested new methodologies for studying carbon fibers.
Keywords
For citations:
Cheblakova E.G., Kleusov B.S., Sapozhnikov V.I., Gorina V.A., Malinina Yu.A., Gareev A.R. Investigation of the properties of high-strength fibers by methods of physico-chemical analysis. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(4):34-40. https://doi.org/10.17073/1997-308X-2023-4-34-40
Introduction
High-strength carbon fibers (CF) find a wide range of applications in today’s technological, transportation, and construction industries. The development of these sectors heavily relies on the utilization of fibrous materials, and CFs are a key component in the production of composite materials. They are primarily derived from various polymer fibers, with polyacrylonitrile (PAN) fibers being the most common choice. PAn fibers offer exceptional properties, such as high strength, a relatively high modulus of elasticity, low specific gravity, and the ability to withstand high temperatures without weight loss. These characteristics make CFs valuable in various applications. Over the last few decades, a significant body of scientific and technical literature [1–18] has been dedicated to this subject. However, some physico-chemical parameters’ impact on the final properties of CF remains inadequately explored.
The objective of this research was to conduct a comprehensive examination of carbon fiber using a combination of analytical techniques, including X-ray phase structural analysis (XPSA), synchronous thermal analysis (STA), atomic emission spectral analysis (AESA), tomographic studies, and the assessment of fiber surface properties. The results obtained from these investigations have been consolidated.
For the experimental studies, UMT 49-12K-EP fiber from Alabuga-Volokno LLC (Umatex JSC) was selected as the starting material.
The data derived from these physico-chemical analytical studies serve as a valuable foundation for the development and proposal of further research methodologies for CF.
Experimental
The image of the fibers was obtained using a “SkyScan 1272” (Bruker, Germany) high resolution microtomograph. The scanning was conducted in filterless mode, with parameters set at 50 kV, 200 mA, a rotation step of 0.1°, and a pixel size of 3.81 µm. The reconstruction of the sections was carried out using “NRecon” and “CTvox” software».
For the X-ray phase structural analysis, a D8 Advance diffractometer (Bruker, Germany), which uses Bragg-Brentano geometry, was employed. This device utilized a copper X-ray tube capable of delivering up to 2200 W, generating CuKα radiation with a wavelength of λ = 0.15418 nm. The occurred within the angular range of 2θ = 10÷90°, with an exposure time of approximately 10 min. The fibers were positioned on a silicon cuvette designed to minimize background interference, ensuring even distribution over its surface. Prior to each measurement, the X-ray tube and detector were calibrated. A specialized program, TOPAS, was utilized for the analysis of the diffractograms. The angular position of the diffraction maxima was measured with an absolute error not exceeding ±0.026°.
Atomic emission spectral analysis of CFs was carried out using a DFS-8 (LOMO company, St. Petersburg) in the wavelength range of 220–330 nm. The sample weight used was 7 mg. A glass electrode (type IV) was employed as the lower electrode, while a cone electrode (type I) served as the upper electrode. The fibers were placed in the lower electrode’s crater and lightly dusted with high-purity graphite. The exposure time was set at 10 s, and a 17 A DC arc was employed. Spectra were recorded using a photoelectron cassette, and the analysis was conducted using the SM 2008 program (MORS LLC, Troitsk).
The CF sample also underwent simultaneous thermal analysis using an STA 449 F1 Jupiter device (Netzsch, Germany). The carbon fiber was analyzed under the following conditions:
1) in an argon environment (40 ml/min), with a sample weight of 5 mg. The heating process was uniform, with a rate of 5 °C/min in the temperature range of 25–955 °C, including a 10-min hold time at the maximum temperature;
2) in an air environment (40 ml/min), with a sample weight of 5 mg. Uniform heating at a rate of 5 °C/min took place in the temperature range of 25–950 °С.
The results obtained were processed using the “Proteus Thermal Analysis v.5.1.0” (Netzsch, Germany) software.
This data processing involved the determination of various parameters such as the beginning of weight loss temperatures, residual weight at the final temperature, temperature intervals corresponding to processes with thermal effects (either exo- or endothermic), peak temperature values for thermal effects, and more.
Results and discussion
Figure 1 displays the appearance of the original fiber.
Fig. 1. UMT 49-12K-EP fiber fragment (3D reconstruction) |
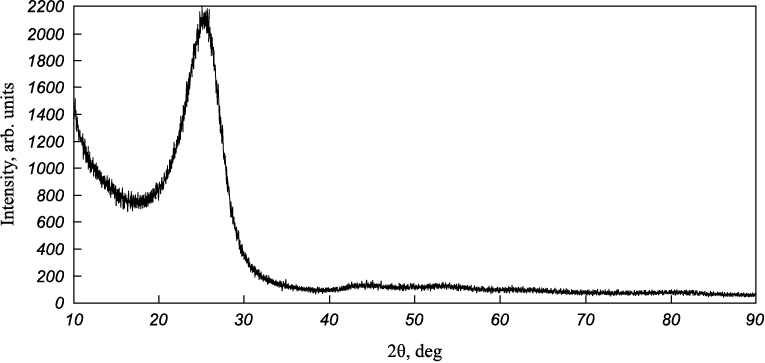
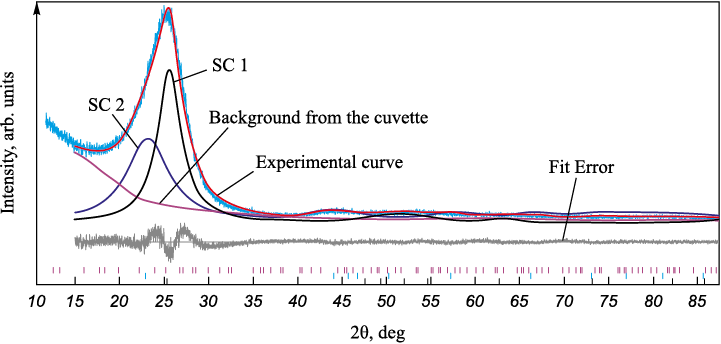
The diffractograms were processed using a two-component profile description model. Figure 2 illustrates a diffractogram of UMT 49-12K-EP carbon fiber, which clearly demonstrates its amorphous nature. Carbon fibers belong to the non-graphitized materials category, meaning that the crystallites are randomly arranged and relatively small compared to graphitizing materials. Notably, there is an asymmetry towards smaller angles, a feature conventionally attributed to the presence of multiple structural components (SCs). In this paper, a two-component model of profile description is chosen (Fig. 3). A component with a larger spacing is referred to as a “kernel,” and a component with a smaller spacing is considered a “shell.” The approximate content of these components, as estimated by the peak areas, is 57 wt. % for SC 1 and 47 wt. % for SC 2.
Fig. 2. Carbon fiber diffraction pattern
Fig. 3. Example of calculation using a two-component model |
The table shows that CF has a large interplanar distance and small crystallite sizes, indicating that this fiber belongs more to amorphous non-graphitic materials.
Data from X-ray phase analysis of UMT 49-12K-EP carbon fiber
| |||||||||||||||
The absolute error of the AESA measurements was 70–0.5 ppm.
The results of atomic emission spectral analysis for the UMT 49-12K-EP sample are displayed below, ppm:
| ||||||||||||||||||||||||||||||||||||||||||
The content of impurities in carbon fiber was determined in accordance with MI00200851-323-2009 (a procedure by JSC “NIIgraphite”). Silicon was identified as the primary contributor to the total impurity content. The presence of silicon is attributed to the addition of an organic compound containing silicon during the precursor’s production phase for subsequent catalytic graphitization of hydrocarbons. Further details regarding the presence of Si in high-strength carbon fibers are elaborated upon in reference [19].
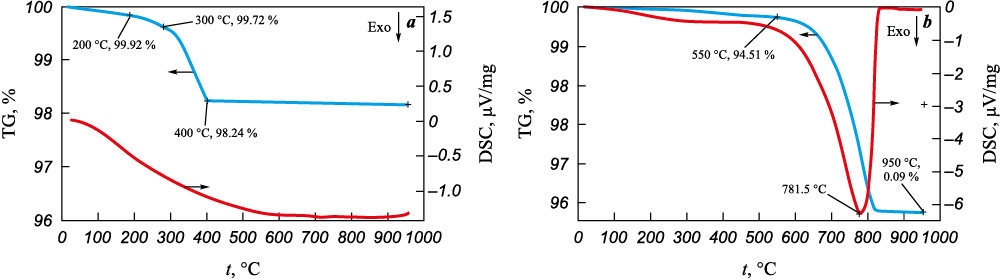
The results of thermal analysis of CFs are presented in the form of graphs displaying TG and DSC signals versus temperature (Fig. 4).
Fig. 4. Results of simultaneous thermal analysis of UMT 49-12K-EP carbon fiber |
In an inert environment, as depicted in Figure 4, a, the CF sample initiates weight loss at temperatures exceeding 100 °C. At around 300 °C, a 0.3 % weight loss occurs, attributed to the gradual removal of residual moisture and volatile substances. Subsequently, the weight loss rate escalates significantly as a result of the thermal degradation of the epoxy sizing agent applied to the carbon fiber. This sizing agent serves to enhance the wettability and adhesion of polymer binders to the CF surface when producing composite materials. The drop in weight, about 1.5 % in the temperature range of 300÷400 °C aligns with the sizing agent’s manufacturer-declared content in hydrocarbons (1.2–1.7 wt. %). Upon further heating in argon, weight loss essentially ceases (<0.1 wt. % at 400÷955 °C). The residual weight of CF at the final temperature is 98.15 wt. %, consistent with published data suggesting that, in an inert environment, carbon fiber can withstand heating above 1000 °C without altering its mechanical properties [3]. This high thermal resistance is achieved through the rigorous temperature treatment during the production of carbon fiber.
In the presence of atmospheric oxygen, when the temperature reaches 550 °C, the CF sample experiences a loss of over 5 % of its weight. As the temperature continues to rise, active oxidation (combustion) of the sample takes place, marked by a significant exothermic effect (a peak on the DSC curve at 781.5 °C). In previous studies [1; 3], it has been noted that in an air environment, the maximum operational temperature for carbon fiber without additives, before thermal oxidation initiates, ranges from 300–370 °C. Given that the residual weight at 950 °C is approximately 0.09 wt. % (equivalent to 900 ppm), in line with the earlier AES results (Σimpurities = 909 ppm), it can be inferred that the unburned residue comprises impurities such as silicon compounds, calcium, and other elements present in small quantities within the carbon fiber composition. These compounds appear to contribute to increasing the thermal oxidative stability of CF in the presence of air.
The surface properties of UMT49-12K-EP fiber were examined using nitrogen adsorption with an ASAP 2020 device (Micromeritics, USA). The specific surface area was determined using the BET method, while the volume of mesopores (diameter <900 Å) and their size distribution were calculated using the Barrett, Joyner, and Halenda (BJH) method across a pressure range of 0.35–0.95 p/ps .
The CF surface properties are as follows:
Ssp , m2/g . . . . . . . . . . .0.29
Vp , сm3/g . . . . . . . . . . 0.0002
Dp , Å . . . . . . . . . . . . . 255
where Ssp is the specific surface area, Vp is the relative volume of mesopores, Dp is their average diameter.
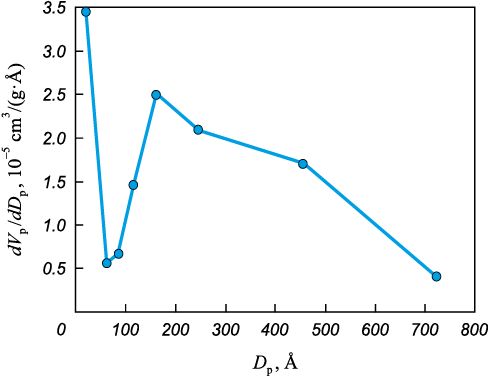
Figure 5 displays the mesopore size distribution. The graph of relative pore volume against their diameter exhibits distinct peaks, indicating the presence of groups of pores of similar size.
Fig. 5. Relative volume of mesopores as an integral function of their diameter |
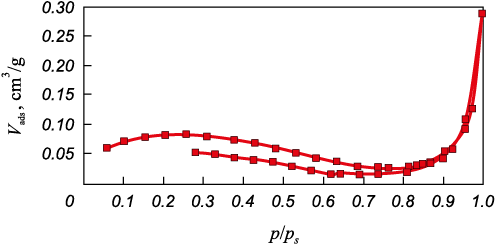
Figure 6 depicts the isotherm of the carbon sample under study. It falls into the 4\(^{{\rm{th}}}\) type of isotherms, following the BDDT international classification, which is characteristic of non-porous materials, specifically PAN fiber. On the adsorption branch, there’s a consistent increase in specific sorption (Vads) as the relative index p/ps , rises, followed by a sharp increase at around p/ps = 1. The isotherm exhibits an extended and irreversible hysteresis.
Рис. 6. Nitrogen adsorption-desorption isotherm |
The determination of surface functional group content in the carbon fiber was conducted following the MI-00200851-331-2010 procedure (JSC NIIgraphfit). The analysis revealed the absence of carboxyl, phenolic, and carbonyl groups.
Based on the data acquired, we can deduce that the fiber’s surface lacks activation and doesn’t possess acid-base centers. In terms of its surface properties, the fiber aligns with the class of carbon fibers derived from PAN precursor [20–22]. It exhibits adsorption and chemical inactivity, which could further delineate its potential application.
Conclusions
1. The structural characteristics of carbon fiber have been thoroughly examined, and its chemical composition has been detailed. It has been conclusively determined that carbon fiber possesses an underdeveloped surface and does not contain surface carboxyl, phenolic, and carbonyl groups.
2. The predominant impurities in the chemical composition of carbon fiber have been identified as silicon compounds.
3. Precise temperatures for the thermal degradation of the carbon fiber sizing agent in an inert environment (300–400 °C) have been ascertained. The carbon fiber itself demonstrates exceptional heat resistance, enduring temperatures up to 950 °C without any loss of weight.
4. It has been established that the presence of elemental organic compounds in the composition of CF contributes significantly to an increase in thermal oxidative stability. Oxidation (combustion) commences at temperatures exceeding 550 °C, in contrast to the 350 °C threshold for CF without additives.
5. This research suggests new physico-chemical methods for investigating carbon fiber.
References
1. Fialkov A.S. Carbon-graphite materials. Moscow: Energiya, 1979. 320 p. (In Russ.).
2. Aizenshtein E.M., Klepikov D.N. Polyester fibers: today and tomorrow. Vestnik Khimicheskoi Promyshlennosti. 2016;91(4):6–10. (In Russ.).
3. Simamura S. Carbon fiber. Moscow: Mir, 1987. 304 p. (In Russ.).
4. Kablov E.N. Innovative developments of FSUE “VIAM” SSC of RF on realization of “Strategic directions of the development of materials and technologies of their processing for the period until 2030”. Aviatsionnye Materialy i Tekhnologii. 2015;34(1):3–33. (In Russ.). https://doi.org/10.18577/2071-9140-2015-0-1-3-33
5. Meleshko A.I., Semenov V.I., Shaidurov V.S. Manufacture of carbon fibres and plastics based on them. GONTI-25. 1992;VIII(60):49. (In Russ.).
6. Tyumentsev V.A., Fazlitdinova A.G. Study of fibrous carbon materials using X-ray diffractometry. Zavodskaya laboratoriya. Diagnostika materialov. 2019;85(11):31–36. (In Russ.).
7. Meleshko A.I., Polovnikov S.P. Carbon, carbon fibers, carbon composites. Moscow: Science Press, 2007.189 p. (In Russ.).
8. Kablov E.N. Composites: today and tomorrow. Metally Evrazii. 2015;1:36–39. (In Russ.).
9. Sidorina A.I., Gunyaeva A.G. Market of carbon products and composites based on them. Khimicheskie Volokna. 2016;4:48–53. (In Russ.).
10. Bennett S.C., Johnson D.J., Johnson W.J. Strength-structure relationships in PAN-based carbon fibres. Journal of Materials Science. 1983;18:3337–3347.
11. Huang X. Fabrication and properties of carbon fibers. Materials. 2009;2:2369–2403.
12. Hu X., Wang L., Xu F., Xiao T., Zhang Z. In situ observations of fractures in short carbon fiber/epoxy composites. Carbon. 2014;67:368–376. https://doi.org/10.1016/j.carbon.2013.10.007
13. Goodhew P.J., Clarke A.J., Bailey J.E. Review of fabrication and properties of carbon-fibers. Materials Science and Engineering. 1975;17(1):3–30. https://doi.org/10.1016/0025-5416(75)90026-9
14. Panin M.I., Kapustin V.M., Tsymbalyuk A.E. The use of complex filaments for reinforcement of fibrous composite materials used in the oil and gas industry. Izvestiya Vuzov. Tekhnologiya Tekstilnoi Promyshlennosti. 2021;(6):103–106. (In Russ.).
15. Gunyaeva A.G., Sidorina A.I., Kurnosov A.O., Klimenko O.N. Polymeric composite materials of new generation on the basis of binder VSE-1212 and the filling agents alternative to ones of Porcher Ind. and Toho Tenax. Aviatsionnye Materialy i Tekhnologii. 2018;3(52):18–26. (In Russ.). https://doi.org/10.18577/2071-9140-2018-0-3-18-26
16. Morgan P. Carbon fibers and their composites. London: Taylor and Francis. 2005. 1200 p.
17. Park S.-J. Carbon fibers. 2nd ed. Springer, 2018. 366 p. (Springer Series in Materials Science, Vol. 210).
18. Frank E., Steudle L.M., Ingildeev D., Spörl J.M., Buchmeiser M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angewandte Chemie International Edition in English. 2014;53(21):5262–5298. https://doi.org/10.1002/anie.201306129
19. Verbets D.B., Samoilov V.M., Bubnenkov I.A., Buchnev L.M., Nakhodnova A.V., Stepareva N.N. Change in the structure and properties of carbon fibers during graphitazion using an exstract or a hologencontaining medium. In: The Research Institute of Graphite-based Structural Materials is 60 years old. Moscow: Nauchnye tekhnologii, 2020. P. 86–102. (In Russ.).
20. Beck N.V., Meech S.E., Norman P.R., Pears L.A. Characterisation of surface oxides on carbon and their influence on dynamic adsorbtion. Carbon. 2002;40:531–540. https://doi.org/10.1016/S0008-6223(01)00144-0
21. Ho K., Qian H., Bismarck A. Carbon fiber: Surface properties. In: Wiley Encyclopedia of Composites. John Wiley & Sons, Inc., 2011. P. 1–11. https://doi.org/10.1002/9781118097298.weoc024
22. Martínez-Alonso A., Jamond M., Montes-Morán M.A., Tascón J.M.D. Microporous texture of activated carbon fibers prepared from aramid fiber pulp. Microporous and Mesoporous Materials. 1997;11:303–311. https://doi.org/10.1016/S0927-6513(97)00050-3
About the Authors
E. G. CheblakovaRussian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
B. S. Kleusov
Russian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
V. I. Sapozhnikov
Russian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
V. A. Gorina
Russian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
Yu. A. Malinina
Russian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
A. R. Gareev
Russian Federation
2 Bld. 1, Electrodnaya Str., Moscow 111524, Russia
Review
For citations:
Cheblakova E.G., Kleusov B.S., Sapozhnikov V.I., Gorina V.A., Malinina Yu.A., Gareev A.R. Investigation of the properties of high-strength fibers by methods of physico-chemical analysis. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(4):34-40. https://doi.org/10.17073/1997-308X-2023-4-34-40