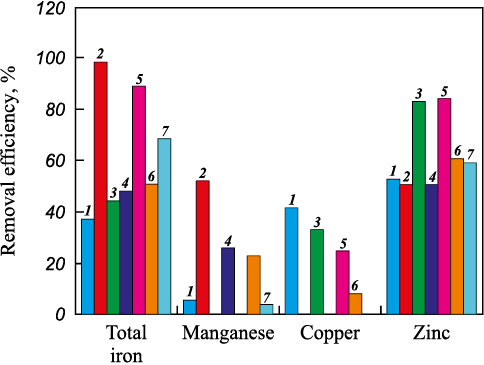Scroll to:
Removal of heavy metal ions from industrial (mining) wastewater using electromagnetically activated carbonaceous sorbent
https://doi.org/10.17073/1997-308X-2024-2-35-44
Abstract
Mining wastewater, characterized by elevated salt levels, necessitates effective treatment to prevent contamination of underground and surface water. Traditional methods for treating large volumes of mining wastewater with high total dissolved solids are expensive, and cost-effective alternatives are limited. In this study, we propose a solution to this challenge: the sorption of dissolved substances using a carbonaceous sorbent derived from waste, specifically rice husk biochar. To enhance the sorbent’s efficiency, we subjected it to electromagnetic activation, resulting in increased carbon content (from 43.3 to 78.5 % compared to the initial biochar), reduced impurities, and particle size reduction to the nanoscale (1–50 nm) with the formation of mesopores (mean diameter from the adsorption isotherm is 167 Å) and micropores (4.92 Å). This process contributes to improved compositional homogeneity. The effectiveness of the proposed sorbent was validated through the treatment of wastewater from Kirov Mine (Novoshakhtinsk, Rostov Region) under laboratory conditions. The removal rates for dissolved heavy metal ions (iron, zinc, manganese) were found to be 89, 84 and 26 %, respectively. A recommended two-stage sorption treatment involves: (1) static sorption using electromagnetically treated rice husk biochar at a concentration of 0.5 g/dm3; (2) subsequent reagent treatment of the suspension (SKiF-180 reagent, 1.0 mg/dm3), addition of potassium permanganate for manganese removal, settling for 30 min, and non-pressure filtration with a rice husk biochar filter.
Keywords
For citations:
Smolyanichenko A.S., Yakovleva E.V. Removal of heavy metal ions from industrial (mining) wastewater using electromagnetically activated carbonaceous sorbent. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(2):35-44. https://doi.org/10.17073/1997-308X-2024-2-35-44
Introduction
Discharge of highly concentrated mining wastewater into the environment poses a significant hazard due its elevated levels of heavy metal ions, including iron, manganese, zinc, nickel and others, as well as high total dissolved solids (5.0–15.0 g/dm3) [1; 2].
Groundwater contamination by heavy metal ions, such as soluble Fe\(^\rm{2+}\) and Mn(II) compounds, further exacerbates the environmental impact. Russian environmental standards specify maximum allowable concentrations (MAC) for iron (0.3 mg/dm3) and manganese (0.1 mg/dm3) in drinking water [3; 4].
It is essential to recognize that the treatment of mine wastewater is a multifaceted process. The initial stage involves mechanical treatment, including clarification, filtration, and the separation of solid particles through centrifugal forces. Subsequent stages encompass chemical processes (coagulation, flocculation, sorption, neutralization, decontamination), physical treatments (ultrasonic, UV, magnetic exposure), and biological treatments [5; 6]. A mandatory step in the treatment of mine and quarry wastewater involves decontamination through chemical methods (ozonation) and physical methods (UV) before discharge into the environment [7–9].
This study proposes a sorption process for removing heavy metal ions dissolved in wastewater. The process utilizes a carbonaceous sorbent derived from agro-industrial waste and subjected to electromagnetic treatment. The suggested solution can be applied in mining wastewater treatment plants.
Materials and methods
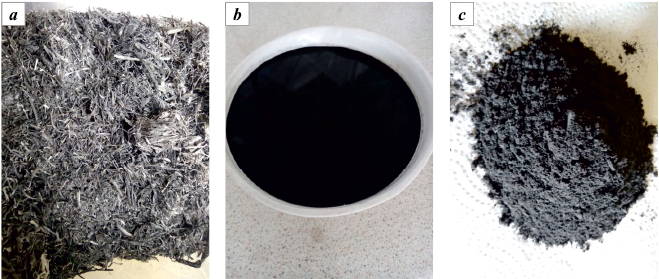
We utilized biochar derived from rice husks (rice straw) as the carbonaceous sorbent. This biochar was produced by carbonizing pre-washed rice husks in a muffle furnace at 600 °C for 30 min, followed by treatment in an activator (Fig. 1). A portion of the biochar was then mixed in distilled water, transferred to a non-magnetic cylinder containing ferromagnetic particles with a weight of m = 200 g, and subjected to a rotating electromagnetic field for 30 s within the activator. Subsequently, it was dried in a desiccator for 4 h at t = 105 °С (Fig. 2).
Fig. 1. The activator
Fig. 2. The sorbent at each stage of preparation |
The rotation of ferromagnetic particles in the electromagnetic field induces a magnetostrictive effect, leading to the reduction of oxides on the particle surface. This process results in an increase in the carbon content of the sorbent from 43.3 to 78.5 %, as compared to the initial biochar, and a reduction in impurities, including silicon, from 8.2 to 2.1 % (refer to Table 1).
Table 1. Chemical composition of rice husk biochar samples
| |||||||||||||||||||||||||||||||||||||||||||
The alterations in the chemical composition of biochar stem from various processes occurring within the activator. The increase in carbon content can be attributed to the disruption of intermolecular bonds [10]. Additionally, the interaction of SiO2 with ferromagnetic particles leads to the formation of chemical compounds on their surface layer, resulting in a reduction in silicon content [11]. The activation process not only pulverizes the sorbent into the nanoscale range (1–50 nm) but also generates mesopores (with a mean diameter of 167 Å) and micropores (4.92 Å) while enhancing the homogeneity of the composition. Following the preparation of the carbonaceous sorbent as described above, we assessed its effectiveness for mining wastewater treatment under laboratory conditions.
Table 1 presents the properties of the initial biochar derived from rice husks and the biochar following electromagnetic activation 1.
We conducted thermogravimetric analysis (TGA) of the sorbent, and the summarized results are provided below:
|
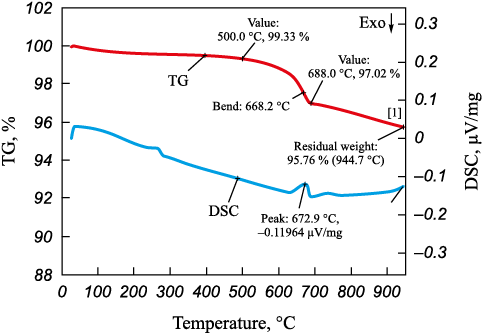
The sorbent sample, post-activation, underwent calcination at t = 450 °С for 3 h. During this process, its color transitioned from black to brown, and there was a slight reduction in volume. The observed 0.7 % loss upon heating to 450 °C suggests the potential presence of a minimal amount of moisture absorbed during sample preparation. Given the requirement for a very small sample size (less than 20 mg) in thermal analysis, even a minor influence from ambient air moisture during sample preparation could impact the results. Upon heating to 500 °C, the calcined sample experienced almost negligible weight loss. In the temperature range of 500 to 688 °C, a weight loss of approximately 3.3 % occurred, as indicated by the endothermic effect in the DSC curve (Fig. 3).
Fig. 3. TGA derivatogram of the sorbent sample 1 |
The observed process is likely analogous to transformations occurring at t = 620÷685 °C, involving the release of chemically bound moisture and some organic volatiles. Continued heating to 950 °C led to a gradual decline in the rate of weight loss. The residual weight at 950 °C was determined to be 95.76 %. Notably, the TGA curve does not reach a plateau, indicating ongoing decomposition of the sample.
In this study, the wastewater sourced from the Kirov mine (Novoshakhtinsk, Rostov Region) was employed. The mine generates an estimated daily volume of approximately 40,000 m3 of wastewater, which is pumped from the mine and directed to the primary settling basin for the removal of suspended solids. Despite undergoing clarification, the treated wastewater remains unsuitable for discharge into the environment, leading to the imposition of environmental pollution penalties.
An illustration of the mine wastewater composition post-treatment at the existing treatment plant is provided in Table 2. Notably, this composition does not adhere to the stipulated environmental requirements [12; 13].
Table 2. Composition of mine wastewater before and after treatment
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Our investigation encompassed both the steady-state and dynamic reduction of dissolved heavy metal ion concentrations, specifically iron, manganese, copper, and zinc, under laboratory conditions. In static conditions, a fluid element remains stationary relative to the sorbent particle, indicating that they move together. Conversely, in dynamic conditions, the fluid element moves relative to the sorbent particle, with the absorbed substance present in a mobile liquid phase that is filtered through the sorbent layer.
Condition 1. Static sorption conditions:
– introduction of rice husk biochar, subjected to electromagnetic treatment, into the original mine wastewater at various amounts (0.1; 0.3; 0.5; 0.7 and 1.0 g/dm3), followed by mixing in a flocculator at 45 rpm for 30 min;
– treatment of the resulting suspension with the SKiF-180 reagent, consisting of aluminum polyoxychloride as the coagulant and polydiallyldimethylammonium chloride (PolyDADMAC) as the cationic flocculant, at a dosage of 1.0 mg/dm3. This treatment involves stirring for 2 min at 200 rpm and an additional 10 min at 45 rpm;
– allowing the wastewater to settle for 30 min to facilitate coagulation;
– filtration through a quartzite pressure filter.
Condition 2. Dynamic sorption conditions (filtration):
– treatment of the initial wastewater with the SKiF-180 reagent (1.0 mg/dm3) through stirring for 2 min at 200 rpm and an additional 10 min at 45 rpm;
– settling the wastewater for 30 min to induce coagulation;
– filtration through a non-pressure filter containing rice husk biochar particles sized 1–3 mm.
Results and discussion
All mine drainage samples collected during testing were analyzed by a certified laboratory, following standard test procedures for total iron (PND F 14.1:2:4.50-96), manganese (PND F 14.1:2:61-96), copper (PND F 14.1:2:4.48-96), and zinc (PND F 14.1:2:4.60-96). The laboratory employed a UNICO 1201 spectrophotometer (UNITED PRODUCTS & INSTRUMENTS, USA).
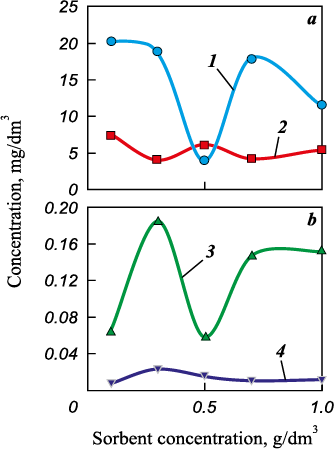
Table 3 presents the concentrations of the mentioned chemical elements in mining wastewater after static sorption using rice husk biochar (condition 1). A notable reduction in iron ion concentration, ranging from 44 to 89 % compared to the initial concentration, was observed. The optimal treatment efficiency was achieved at a sorbent concentration of 0.5 g/dm3. The largest decrease in zinc ion content, by 84 %, resulted in a concentration of 0.059 mg/dm3. A marginal drop (4–26 %) in manganese ion concentration occurred when 0.3, 0.7 and 1.0 g/dm3 of the sorbent were added, while in other cases, the concentration increased. Therefore, it is recommended to oxidize manganese using potassium permanganate. To remove 1.0 mg of Mn(II), 1.88 mg of KМnО4 is required. As the initial copper ion concentration in the wastewater is below the rated threshold, evaluating copper removal efficiency is not feasible.
Table 3. Results of mining wastewater treatment under Condition 1
| ||||||||||||||||||||||||||||||||||||||||||||||
Fig. 4 depicts concentration curves illustrating the variation of controlled chemical elements (iron, manganese, zinc, and copper) in relation to the amount of sorbent (rice husk biochar after electromagnetic treatment).
Fig. 4. Concentrations of iron and manganese (a), |
Throughout the tests, we monitored the following parameters:
– redox potential, serving as an indicator of the substance’s ability to accept or donate electrons (oxidation-reduction capability);
– total dissolved solids, quantifying the amount of salts dissolved in water, measured using a TDS-3 meter, and pH levels.
The summarized results of these measurements are presented in Table 4 and visually represented in Fig. 5.
Table 4. Results of mining wastewater treatment under Condition 1
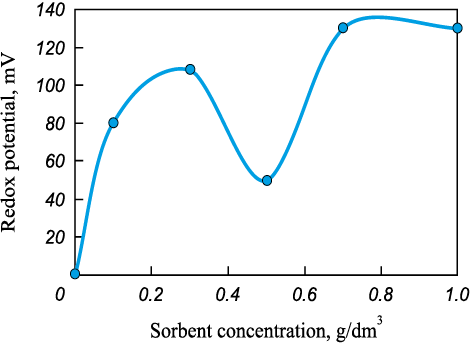
Fig. 5. Redox potential of water vs. sorbent amount | ||||||||||||||||||||||||||||||
It is established that when a certain critical threshold of the hydrogen ion concentration (pH) is reached, doubly and triply charged simple and hydrolyzed cations react to form practically or poorly soluble metal hydroxides [14]. However, the measured pH values fall within the range of 6.15 to 6.39, indicating a neutral environment. The total dissolved solids (TDS) range from 258 to 271 mg/dm3, which falls within the category of low TDS, resembling potable water conditions [15]. Notably, these TDS values exhibit independence from the amount of sorbent used. Given these findings, it can be inferred that a broader range of values for pH and TDS may be necessary to establish a relationship with the amount of sorbent.
Upon introducing the coagulant (SKiF-180 reagent with рН = 0.5÷3.0), the redox potential shifts from negative to positive, signifying a transition from reducing to oxidizing water properties. With an increase in sorbent quantity, the water’s oxidizing properties intensify. However, at a biochar dose of 0.5 mg/dm3, the redox potential decreases from +108 to +50 mV. This reduction is attributed to the decline in iron concentration at this specific sorbent quantity (Fig. 5).
Using iron as an example, we calculated its removal efficiency (E, %) and the sorption capacity of the sorbent (А, mg/g) using the following formulas:
| \[A = \frac{{({C_0} - C)V}}{m},\] | (1) |
where С0 and С are the initial and equivalent concentrations of iron, mg/dm3; V is the volume of the adsorbate volume, dm3; m is the weight of the sorbent, g;
| \[E = \frac{{{C_0} - C}}{{{C_0}}} \cdot 100{\rm{ }}\% ,\] | (2) |
where С0 and С are the iron concentrations in the initial and treated wastewater, mg/dm3.
The results of these calculations are presented in Table 5.
Table 5. Sorption capacity and iron
| ||||||||||||||||||||||||||
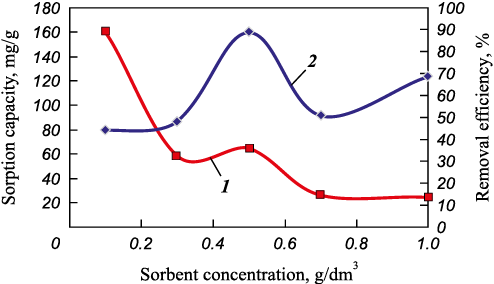
Based on these computations, we generated curves illustrating the sorption capacity and iron removal efficiency as functions of the amount of sorbent (Fig. 6).
Fig. 6. Sorption capacity (1) and iron removal efficiency (2) vs. sorbent amount |
The results from tests conducted under Condition 2 are presented in Table 6. The most effective removal was observed for divalent iron, with an efficiency of 98 %, leading to a residual concentration of 0.6 mg/dm3. While this slightly exceeds the maximum allowable concentration, it suggests that a two-stage sorption process may be advisable. Manganese removal efficiency from the initial mining wastewater reached 52.07 %, meeting the maximum allowable concentration of 5.619 mg/dm3 (actual concentration: 2.693 mg/dm3). As the copper concentration in the initial wastewater is below the threshold, evaluating copper removal efficiency is not feasible.
Table 6. Results of mining wastewater treatment under Condition 2
|
Analyzing the obtained data, the following observations can be made. After coagulating the initial wastewater with the acidic SKiF-180 reagent (pH = 0.5÷3.0), the redox potential of the solution changes its sign from “–” to “+”, indicating oxidizing properties. The pH value of the water experiences a slight change from 6.76 to 6.35, remaining within the rated range. Following filtration, the redox potential further increases from +73 to +215, along with a rise in the pH value (8.47), corresponding to a slightly alkaline environment.
As previously mentioned, the optimal sorbent amount for iron and zinc removal is 0.5 mg/dm3. However, when increased to 0.7 mg/dm3, the efficiency of biochar sharply decreases. Iron removal efficiency drops from 89.06 to 50.86 % (Fig. 4, a and Fig. 6), and zinc removal efficiency drops from 84.31 to 60.9 % (Fig. 4, b). A similar drop in zinc removal efficiency is observed with increasing sorbent dose in the 0.1 to 0.3 mg/dm3 range. This phenomenon might be attributed to a reduction in the effective specific surface area accessible to metal ions due to overlapping or aggregation of adsorption centers, thereby increasing the diffusion path length for these ions [16]. This aggregation tends to increase with the weight of the sorbent. Another plausible explanation is that a higher quantity of sorbent provides more active adsorption centers, causing them to remain unsaturated after adsorption [17–19].
Using equation (2), we estimated the removal efficiency for iron, manganese, copper, and zinc under static conditions using rice husk biochar as the sorbent in a range of concentrations, followed by electromagnetic treatment in condition 1 and filtration in condition 2 (Fig. 7).
Fig. 7. Removal efficiency at different stages of mining wastewater treatment |
Conclusions
In summary, the following conclusions can be drawn.
1. The proposed electromagnetic activation of rice husk biochar [20] demonstrates efficiency in treating mining wastewater under laboratory conditions.
2. The sorbent exhibits a chemical composition that is comparable to activated carbon, widely utilized in water treatment, affirming the applicability of the sorbent in water treatment plants.
3. To optimize the efficiency of mining wastewater treatment, we recommend a two-stage sorption process: static sorption utilizing electromagnetically treated rice husk biochar at a concentration of 0.5 g/dm3 (Table 3) (Stage 1), followed by reagent treatment of the suspension (SCiF-180 reagent, 1.0 mg/dm3), addition of potassium permanganate for manganese removal, settling for 30 min, and non-pressure filtration with a rice husk biochar filter (dynamic sorption, Stage 2).
4. Implementation of this process in mining wastewater treatment facilities is anticipated to reduce the concentrations of dissolved heavy metal ions, particularly iron, zinc, and manganese, below the maximum allowable concentrations, enabling the discharge of treated water into the environment.
References
1. Wright I.A., Paciuszkiewicz K., Belmer N. Increased water pollution after closure of Australia’s longest operating underground coal mine: a 13-month study of mine drainage. Water Chemistry and River Ecology Water. Air Soil Pollut. 2018;229(55):1–20. https:/doi.org/10.1007/s11270-018-3718-0
2. Phuong T.D., Vu C.D. Mine water treatment in Hongai coal mines. E3S Web of Conf. 2018;35(01007):1–5. https://doi.org/10.1051/e3sconf/20183501007
3. Arefieva O.D., Shapkin N.P., Gruschakova N.V., Prokuda N.A. Mine water: Chemical composition and treatment. Water Practice and Technology. 2016;11(3):540–546. https:/doi.org/10.2166/wpt.2016.060
4. Kulikova A.A., Sergeeva Yu.A., Ovchinnikova T.I., Xabarova E.I. Formation of mine water composition and analysis of treatment methods. MIAB. Mining informational and analytical bulletin. 2020;7:135–145. (In Russ.). https:/doi.org/10.25018/0236-1493-2020-7-0-135-145
5. Maksimovich N.G., Pyankov S.V., Khayrulina E.A. Environmental assessment of closeded coal mine territory using G1S analysis. Mine Water and Circular Economy Mine Water and Circular Economy. 2017;212–217.
6. Tarasenko I.A., Zin`kov A.V. Assessment of the environmental safety of the underground water basin during the closure of the mines of Primorsky Krai (on the example of the Lipovetskaya mine). Mining informational and analytical bulletin. 2013;2:362–373. (In Russ.).
7. Budykina T.A. Treatment of wastewater resulting from iron ore beneficiation. Magazine of Civil Engineering. 2018;6(82):163–169. (In Russ.). https:/doi.org/10.18720/MCE.82.15
8. Gubina N.A., Ylesin M.A., Karmanovskaya N.V. Ways to increase the productivity and quality of mine water treatment. Journal of Environmental Management and Tourism. 2018;9(3):423–427. https://doi.org/10.14505//jemt.v9.3(27).03
9. Stavitskaya S.S., Goba V.E., Petrenko T.P., Kovtun M.F., Kartel’ N.T. Industrial wastewater treatment using modified anthracite and other carbon sorbents. Khimiya tverdogo topliva. 2003;2:56–62. (In Russ.).
10. Rempel’ A.A., Valeeva A.A. Materials and methods of nanotechnology. Yekaterinburg: UrFU, 2015. 136 p. (In Russ.).
11. Serzhanov G.M., Shevko V.M., Lavrov B.A., Amanov D.D. Thermodynamic modeling of silicon reduction from aluminum oxide. Mezhdunarodnyi zhurnal prikladnykh i fundamental’nykh issledovanii. 2015;11(2): 161–166. (In Russ.). https://applied-research.ru/ru/article/view?id=7698 (accessed: 03.03.2024).
12. Sanitary norms and rules 2.1.3684-21 “Sanitary and epidemiological requirements for the maintenance of territories of urban and rural settlements, for water bodies, drinking water and drinking water supply, atmospheric air, soils, residential premises, operation of industrial, public premises, organization and conduct of sanitary and anti-epidemic (preventive) measures”, 2021. (In Russ.). URL: https://www.rospotrebnadzor.ru/files/news/SP2.1.3684-21_territorii.pdf (accessed: 01.02.2023).
13. Hygienic standards 2.1.5.1315-03 Maximum permissible concentrations (MPC) of chemicals in the water of water bodies of economic and drinking and cultural and domestic water use. Moscow: Ministry of Health of Russia, 2003. (In Russ.). URL: https://files.stroyinf.ru/Data1/41/41363/index.htm (accessed: 01.02.2023).
14. Vigdorovich V.I., Tsygankova L.E., Protasov A.S. Features of sorption purification of aqueous solutions from heavy metal cations. Message 1. Media with non-hydrolyzable anion. Vestnik TGU. 2013;(1):401–404. (In Russ.).
15. Sanitary norms and rules 2.1.4.1175-02 Hygienic requirements for the quality of non-centralized water supply. Sanitary protection of sources, 2003. (In Russ.). URL: https://files.stroyinf.ru/Data2/1/4294845/4294845751.pdf (accessed: 01.02.2023).
16. Li Y., Xia B., Zhao Q., Liu F., Zhang P., Du Q., Wang D., Li D., Wang Z., Xia Y. Removal of copper ions from aqueous solution by calcium alginate immobilized kaolin. Journal Environmental Science. 2011;23(3):404–411. https://doi.org/10.1016/s1001-0742(10)60442-1
17. Raji C., Anirudhan T.S. Kinetics of Pb (II) adsorption by polyacrylamide grafted sawdust. Indian Journal of Chemical Technology. 1997;4:157–162.
18. Hayrapetyan S.S., Hayrapetyan M.S. A Method for evaluating the sorption capacity of the sorbents. International Journal of modern engineering research (IJMER). 2017;7(6):48–55.
19. Naumenko K., Frolova N., Petrusha O., Chepel N. The method of determination of the sorption capacity of activated carbon by gas chromatography. Eureka: Life Sciences. 2017;(1):12–18. https://doi.org/10.21303/2504-5695.2017.00290
20. Smolyanichenko A.S. Physical and chemical properties of silver-containing nanosorbent obtained from rice straw biochar. Agriculture. 2023;13(7):1288. https://doi.org/10.3390/agriculture13071288
About the Authors
A. S. SmolyanichenkoRussian Federation
Alla S. Smolyanichenko – Cand. Sci. (Eng.), Associate Professor, Water Supply and Disposal Department
1 Gagarin Sqr., Rostov Region, Rostov-on-Don 344003, Russia
E. V. Yakovleva
Russian Federation
Elena V. Yakovleva – Postgraduate Student, Senior Lecturer, Water Supply and Disposal Department
1 Gagarin Sqr., Rostov Region, Rostov-on-Don 344003, Russia
Review
For citations:
Smolyanichenko A.S., Yakovleva E.V. Removal of heavy metal ions from industrial (mining) wastewater using electromagnetically activated carbonaceous sorbent. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(2):35-44. https://doi.org/10.17073/1997-308X-2024-2-35-44