Scroll to:
Evolution of the structural-phase state of steel swarf during its processing into a powdered product
https://doi.org/10.17073/1997-308X-2024-4-6-16
Abstract
Industrial waste recycling is not only linked to significant environmental challenges but also to the recovery of material resources. Typically, these recovered materials are reused within the same technological niche where the waste was generated, often through remelting or adding them to the charge. This study presents an alternative approach that enables the production of a functional powder product from steel swarf during the recycling process, which can subsequently be utilized in the creation of powder metal matrix composites. The initial structure of the swarf, following the turning of a steel billet, was examined using scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis after a processing complex involving additional oxidation and grinding. This analysis aimed to assess the degree of transformation in the structural-phase state of the steel swarf during its processing. It was observed that the swarf post-turning exhibited a complex morphological structure with an uneven distribution of oxygen and carbon. The oxygen present in the initial state of the swarf was insufficient to form a noticeable volume of oxides detectable by X-ray diffraction. However, SEM revealed individual oxide inclusions, each no more than 5 µm in size, located sporadically. Additional oxidation followed by grinding in a vibrating mill altered the structure of the steel swarf, increasing the volume fraction of oxide phases. The study’s findings indicate that the resulting powder from recycled steel swarf is essentially a metal matrix composite with oxide inclusions based on an iron matrix, which holds potential for various future powder technologies.
For citations:
Korosteleva E.N., Nikolaev I.O. Evolution of the structural-phase state of steel swarf during its processing into a powdered product. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):6-16. https://doi.org/10.17073/1997-308X-2024-4-6-16
Introduction
In the framework of resource efficiency and waste utilization strategies, powder metallurgy technologies are highly preferable. This is particularly evident in the recycling of metallic waste within the engineering sector [1–3]. Metal processing on various machines contributes significantly to the total volume of manufacturing waste. Regardless of the processing type and tools used, metal swarf is always produced when manufacturing any part and is often recycled as metal scrap in metallurgical processes [2; 3; 5–7].
Traditionally, metalworking waste is reused either in remelting processes or as a charge for bulk powder blanks and coatings [1; 2; 4–13]. The range of materials studied is broad, encompassing traditional steels, cast irons [5; 6], non-ferrous metals, and alloys [13–15]. Typically, the first stage of recycling metalworking waste involves cleaning it of impurities and contaminants [16–19]. These primarily include organic contaminants from various lubricating-cooling fluids (LCF) and excess oxygen from oxidation processes during machine processing and waste storage [16]. However, these “harmful” impurities and contaminants can be sources of additional elements for forming a functional multicomponent structure in the recycled waste, ultimately transforming it into a powder form.
Several factors motivate this task. First, the swarf structure differs from the original metal billet due to the defect structure formed during cutting [20]. Second, processing modes and environments, including coolant use and oxidation processes, significantly influence it. Third, the swarf is a sufficiently activated material that can undergo additional processing, including oxidation and crushing, to convert it into a powder form [19; 21; 22].
Considering waste from processing steel billets, the resulting swarf can serve as a convenient raw material for preparing powder compositions with a specific combination of components. For example, excess oxygen and oxidation products in the waste from processing steel billets can form oxide inclusions in the steel matrix. The proposed combination of components (Fe + Fe2O3 + Fe3O4 + FeO) can act as a precursor for further use in other multicomponent powder compositions [21–23]. The oxide phases formed after processing steel swarf, along with the iron itself, can actively interact with titanium, aluminum, or other elements [24; 25], creating prospects for developing new metal-matrix composites. Converting steel swarf fragments into powder with a specific phase composition, morphology, and particle dispersion for further use in powder mixtures is essential.
Various methods can grind steel swarf, including electro-physical [8] or simple mechanical methods [19]. Depending on the intended application of the powder blanks, the technological parameters of crushing are determined to obtain powder particles of the required sizes from the recycled waste. Mechanical grinding using crushers or vibratory mills with steel balls is most common [19; 21]. The swarf structure transforms at each processing stage.
Unfortunately, there are currently few studies in this area, making it challenging to predict the possible evolution of the structural-phase state of metalworking waste for further use. If the products of processing steel swarf are considered potential components in powder mixtures, it is interesting to study the evolution of the structure of steel swarf from the initial turning process to converting it into powder form using additional oxidation and grinding. Such analysis is significant in developing new composite materials with oxide inclusions, which was the aim of this study.
Materials and methods
Steel billets made from carbon steel 45, the most common alloy in engineering production, were used as the research material. Its chemical composition according to GOST 1050-2013 is as follows (wt. %:
C . . . . . . . . . . . . . . 0.42–0.50
Si . . . . . . . . . . . . . 0.17–0.37
Cu . . . . . . . . . . . . . ≤ 0.25
As . . . . . . . . . . . . . ≤ 0.08
Mn . . . . . . . . . . . . . 0.50–0.80
Ni . . . . . . . . . . . . . ≤ 0.25
P . . . . . . . . . . . . . . ≤ 0.035
Cr . . . . . . . . . . . . . ≤ 0.25
S . . . . . . . . . . . . . . ≤ 0.04
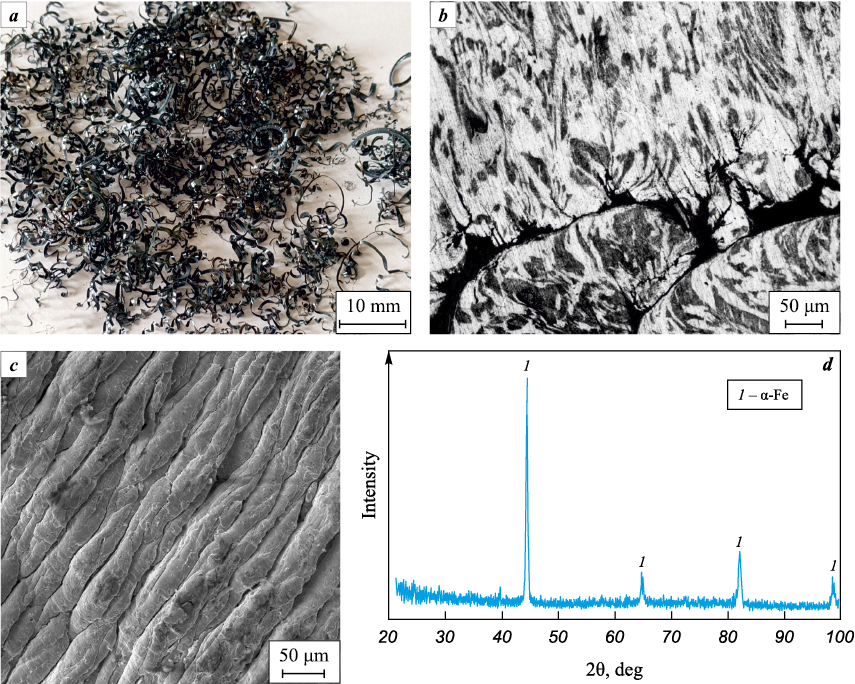
Steel swarf was obtained at a machine-building enterprise after the facing operation without using lubricating-cooling fluids (LCF). Its general appearance in the initial state, microstructure of its fragments after etching with a 4 % aqueous solution of nitric acid, phase composition, and surface morphology after machining are shown in Fig. 1. It is worth noting that the results of X-ray phase analysis of swarf from steel 45 did not reveal lines of oxide phases, showing a practically standard set of phases characteristic of this grade of steel (Fig. 1, d), consistent with previous results [26]. The size of swarf fragments after machining the steel billet was 3–7 mm in width and 10–30 mm in length, necessitating grinding using a vibratory mill designed by ISPMS SB RAS (Russia). Grinding was carried out in an air environment for 10 hours with steel balls of 15 mm diameter at a swarf-to-balls mass ratio of 1:30.
Fig. 1. General appearance of steel swarf (a), microstructure of its fragments (b), SEM image |
Despite the machining conditions and associated processes of temperature fluctuations, oxygen saturation, and work hardening, the swarf remained ductile, complicating the grinding process. Therefore, additional oxidation of the swarf fragments was employed, increasing their brittleness and enabling the production of powder with a wide range of particle dispersity – from 50 to 350 µm. To assess the compressibility and sinterability of the resulting powder, cylindrical samples 10–13 mm high and 10 mm in diameter were pressed and sintered in a vacuum furnace at temperatures ranging
| \[\theta = \left( {1 - \frac{{{\rho _{{\rm{smp}}}}}}{{{\rho _{{\rm{theor}}}}}}} \right) \cdot 100{\rm{ }}\% ,\] |
where ρsmp is the actual density of the sample (g/cm3), and ρtheor is the theoretical density calculated by the additive method for powder particles from the recycled steel swarf, assuming at least 30 vol. % oxide phase and the remainder as a solid solution based on iron corresponding to steel 45.
Since the quantitative values of the oxide fraction are averaged, determining the exact theoretical density is challenging. Therefore, to assess the pore content, the quantitative metallography method was additionally used. The structural-phase changes in the steel swarf were investigated by comparing the results of structural and elemental analyses and the morphology of its fragments in the initial state after turning the steel billet and a set of subsequent actions – oxidation, grinding, pressing, and vacuum sintering.
The studies were conducted using optical metallography, X-ray phase analysis (XRD), and scanning electron microscopy (SEM) with energy-dispersive microanalysis. These analyses were performed using the equipment of the Tomsk Regional Shared-Use Center of NR TSU and the Shared-Use Center of ISPMS SB RAS. The specific equipment used included: optical microscope AXIOVERT-200MAT (Carl Zeiss, Germany), X-ray diffractometers XRD (Shimadzu 6000, Japan) and DRON-8 (NPP Burevestnik, Russia), and scanning electron microscope MIRA 3LMU (TESCAN, Japan).
Results and discussion
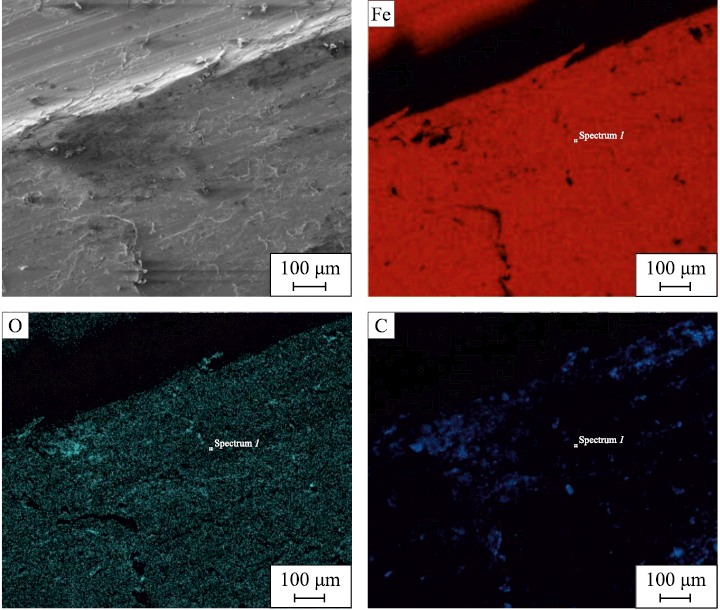
The preliminary analysis of the steel swarf showed that after machining without using lubricating-cooling fluids (LCF), a highly defective structure formed on its surface (Fig. 2). Elemental analysis revealed an uneven distribution of carbon and oxygen (Fig. 2, c, d). The noticeable presence of oxygen on the swarf surface suggests the formation of a certain volume of iron oxides. However, the XRD analysis did not detect oxide phases (Fig. 1, d) in sufficient quantity for identification.
Fig. 2. SEM image of the cut surface and elemental distribution maps of iron, |
It can be assumed that the oxidation processes during initial machining primarily contribute to the formation of amorphous oxide films, which are not visible to XRD, preventing the formation of iron oxide crystallites in significant quantities. Scanning electron microscopy at high magnification revealed individual inclusions that can be attributed to iron oxides based on the elemental distribution map in the local area of the swarf surface (Fig. 3). However, their size and quantity fall below the sensitivity threshold of the X-ray diffractometer.
Fig. 3. SEM image (×10,000) and EDX spectral analysis results of local points |
Since the steel swarf remained ductile after turning, and the oxide phase content was minimal, it was decided to further oxidize the swarf fragments. This aimed not only to increase the iron oxide content but also to enhance the swarf’s brittleness. Thus, the oxidation process would achieve two goals: effective swarf grinding and increased iron oxide content. The simplest methods for this were used: heating in air or soaking in water followed by drying.
Despite the straightforward task of oxidizing carbon steel swarf, the specifics of iron oxidation must be considered. Iron forms several oxides: FeO, Fe2O3 and Fe3O4 . The oxidation process also goes through several stages, where one oxide can transform into another under certain conditions or act as a barrier to its formation. There are also specific temperature regimes that predetermine the formation of a particular group of oxides [27].
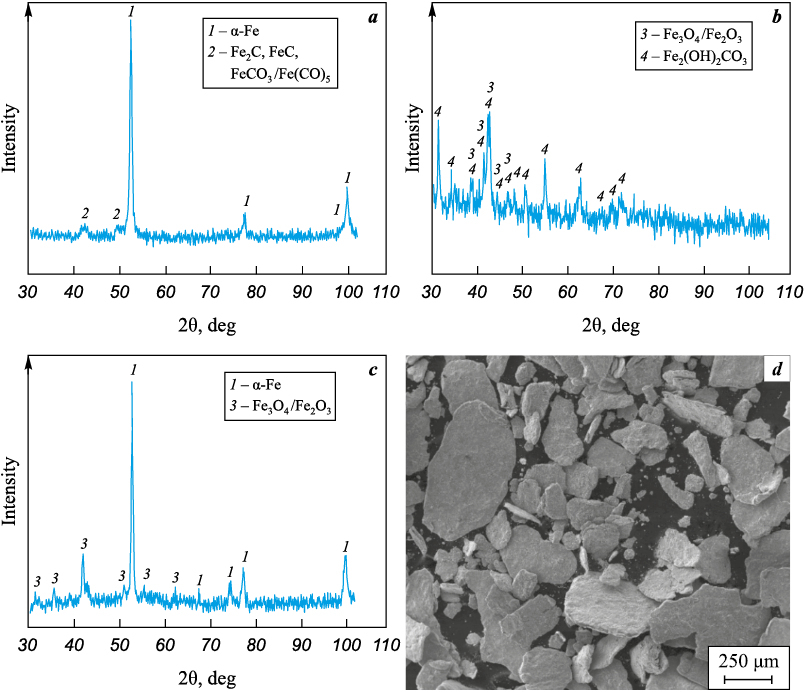
When heating steel swarf in a muffle furnace in air up to 400 °C, XRD results showed that such thermal treatment not only removed the work hardening from machining, which hindered grinding in the vibratory mill but also formed carbide-containing phases based on Fe2C, FeC, FeCО3 or Fe(CО)5 (Fig. 4, а). Since the lines of these phases were few and their intensity very weak, correctly identifying these phases is challenging and requires separate investigation. It is assumed that a mixture of carbide or oxycarbide phases primarily formed on the surface of the swarf fragments may be present.
Fig. 4. Phase composition (a–c) and general appearance (d) of metal ground steel 45 swarf |
The main goal of the experiment was to test different methods to increase the iron oxide content in the steel swarf. Therefore, X-ray phase analysis focused on identifying those oxides not detected by XRD after thermal treatment.
Another oxidation method involved soaking the swarf in water for at least 48 h. Wetting with water followed by air drying at room temperature allowed XRD to detect up to 30–40 vol. % iron oxides within 48 h (Fig. 4, c). There remains a challenge in distinguishing the phases of magnetite Fe3O4 and γ-Fe2O3 by X-ray diffraction, requiring further study. Additionally, an option was explored to obtain powdered fragments from swarf waste by precipitating the suspension of ground steel 45 swarf products from water after evaporation (Fig. 4, b).
After drying the aqueous solution, a fine powder was obtained. Phase analysis revealed a complex configuration of iron dihydroxycarbonate Fe2(OH)2CO3 in the presence of iron oxides. Since this powder product had a very complex composition (requiring separate study) and the productivity of this method was significantly lower than that of the conventional method of soaking in water, drying, and subsequent grinding, further research focused on the sifted powder from the ground swarf after soaking in water.
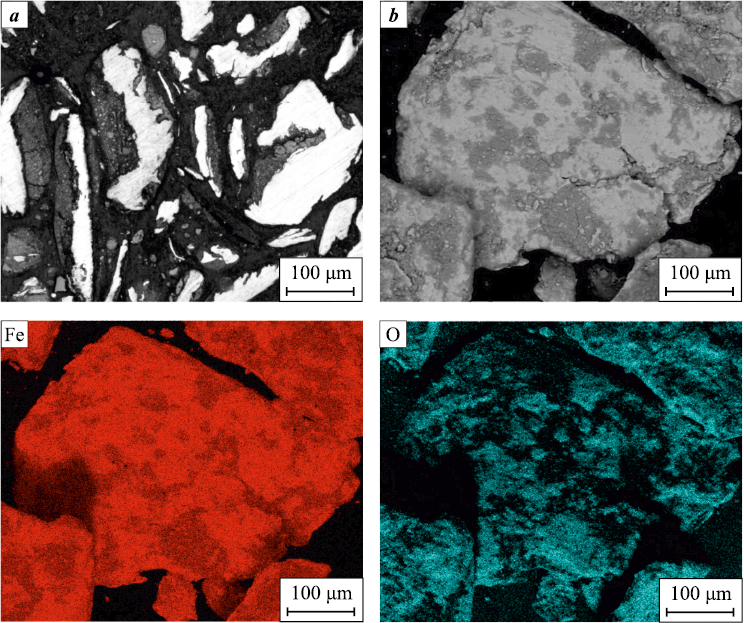
Structural-phase and elemental analyses of the pre-oxidized and ground swarf showed that during the selected comprehensive treatment with oxidation in water and intensive mechanical crushing, the fragments of steel 45 swarf transform into a composite metal matrix powder material (Fig. 5). The particles consist of an α-Fe matrix, with iron oxides Fe2O3 or Fe3O4 as inclusions. It is noteworthy that the oxide phases primarily form on the surface of the ground oxidized swarf fragments (Fig. 5, a), while the original structure is preserved inside the particles.
Fig. 5. Metallographic (a) and SEM (b) image of crushed particles |
The swarf fragmented into particles of a wide size range – from large fragments (300–350 µm) to small ones (20–50 µm). The presence of a significant amount of oxides (at least 30 vol. %) could pose challenges for compacting the obtained powder. Therefore, samples were pressed at a low pressure (350–400 MPa) without adding any plasticizers to test this.
Despite the presence of oxides, the powder was well-compacted, and under the selected pressing load, the residual porosity of the samples was about 40 %. Subsequent vacuum sintering of the pressed samples led to a reduction in porosity due to shrinkage, the intensity of which increased with the sintering temperature (see the Table).
Relative change in volume and porosity of compacts
|
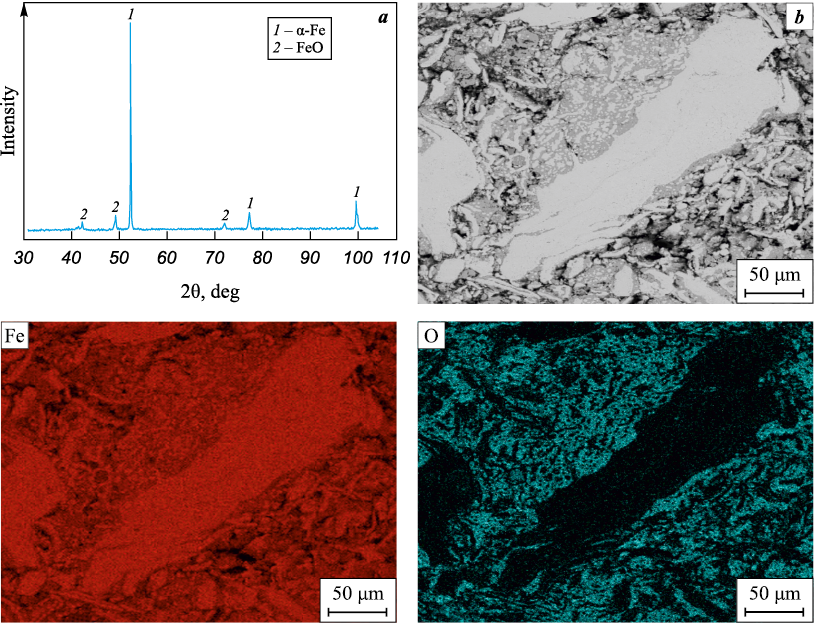
Despite the presence of oxides, the powder was well-compacted, and under the selected pressing load, the residual porosity of the samples was about 40 %. Subsequent vacuum sintering of the pressed samples led to a reduction in porosity due to shrinkage, the intensity of which increased with the sintering temperature (see the Table). The structural-phase state of the powder compact sintered at 1000 °C is shown in Fig. 6. XRD results of the obtained compacts indicated that the formed group of iron oxides Fe3O4 /Fe2O3 transitions to monoxide FeO, with α-Fe being the primary phase (Fig. 6, a). The interparticle contact zones are predominantly filled with oxide components, while the main particle area is almost free of oxygen (Fig. 6). This structural configuration hinders the sintering of fragmented steel swarf particles due to the oxide-containing periphery acting as a barrier. The oxygen-rich extended regions in the recycled swarf powder particles can potentially play an active role in contact interactions with other powder components, such as aluminum or titanium [24], and participate in accompanying reduction reactions or intermetallic synthesis, which is a separate research topic. It is worth noting that the structural-phase state formed in the recycled steel swarf powder could be of interest for other prospective uses [28; 29], especially where the presence of iron oxides is relevant.
Fig. 6. Phase composition (a), microstructure (b) and elemental distribution maps of iron and oxygen |
Conclusions
1. Steel swarf after turning operations on steel 45 billets exhibits specific structural features due to the deformation and physicochemical processes associated with machining. In its initial state, it demonstrates a structure with an uneven distribution of carbon and oxygen, localized in certain areas as fine inclusions within the steel matrix.
2. The XRD analysis of the swarf in its initial state revealed a phase composition identical to that of the steel 45 billet, with the swarf fragments remaining ductile. Additional oxidation of the metalworking waste in water promotes the growth of the oxide phase and facilitates the grinding process of the swarf in a vibratory mill, yielding particles sized 50–350 µm.
3. The analysis of the resulting powders from the oxidized and ground steel 45 swarf in the vibratory mill showed that the powder particles are a metal matrix product with oxide inclusions predominantly in the surface layers. Despite the presence of at least 30 vol. % iron oxides, the powder is well-pressed and sintered, demonstrating volumetric shrinkage and reduced porosity.
4. The elongated regions of oxide phases formed in the ground swarf fragments are active structural inclusions that can interact with additional powder components containing other elements, such as aluminum or titanium. This allows the investigated powder from steel swarf to be considered a potential precursor or oxide-containing component for producing multicomponent metal matrix composites with an oxide phase, utilizing waste from the machine-building industry.
References
1. Rovin S.L., Kalinichenko A.S., Rovin L.E. The return of the dispersed metal waste into production. Litiyo i metallurgiya. 2019;(1):45–48. (In Russ.). https://doi.org/10.21122/1683-6065-2019-1-45-48
2. Ottink T., Vieceli N., Foreman M.R.StJ. Petranikova M. Novel approach to recycling of steel swarf using hydrometallurgy. Resources, Conservation and Recycling. 2022;185:106450. https://doi.org/10.1016/j.resconrec.2022.106450
3. Rovin S.L., Rovin L.E., Zayats T.M., Valitskaya O.M. Recycling of ferrous metal shavings. Litiyo i metallurgiya. 2017;(4):94–101. (In Russ.). https://doi.org/10.21122/1683-6065-2017-4-94-101
4. Bendikiene R., Ciuplys A., Kavaliauskiene L. Circular economy practice: From industrial metal waste to production of high wear resistant coatings. Journal of Cleaner Production. 2019;229:1225–1232. https://doi.org/10.1016/j.jclepro.2019.05.068
5. Dyakonov O.M. Production of metallurgical briquettes on the basis of chips-powder compositions by hot press molding. Litiyo i metallurgiya. 2011;(4):129–137. (In Russ.).
6. Hankel J., Jager S., Weber S. Development of a recycling strategy for grinding sludge using supersolidus liquid phase sintering. Journal of Cleaner Production. 2020;263:121501. https://doi.org/10.1016/j.jclepro.2020.121501
7. Jäger S., Weber S. Upcycling strategy of grinding swarf by super solidus liquid phase sintering. Procedia CIRP. 2020;90:546–551. https://doi.org/10.1016/j.procir.2020.01.079
8. Ageeva E.V., Latypov R.A., Ageev E.V. Evaluation of wear resistance of electrospark coatings fabricated using electroerosion powders of high-speed steel. Powder Metallurgy аnd Functional Coatings. 2015;(1):71–76. (In Russ.). https://doi.org/10.17073/1997-308X-2015-1-71-76
9. Saini S., Singh K. Recycling of steel slag as a flux for submerged arc welding and its effects on chemistry and performance of welds. The International Journal of Advanced Manufacturing Technology. 2021;(114):1165–1177. https://doi.org/10.1007/s00170-021-06866-1
10. Mahmood K., Syed W.U.H., Pinkerton A.J. Innovative reconsolidation of carbon steel machining swarf by laser metal deposition. Optics and Lasers in Engineering. 2011;49:240–247. https://doi.org/10.1016/j.optlaseng.2010.09.014
11. Khlybov A.A., Belyaev E.S., Belyaeva S.S., Getmanovskiy Y.A., Yavtushenko P.M., Ryabtsev A.D., Demchenko A.I. Method for producing article by hot isostatic pressing of carbide steels from chip waste of metal-cutting manufacture: Patent 2775243 (RF) 2022. (In Russ.).
12. Smornov A.G. Method of scrap and iron-and-steel waste packing: Patent 2329311 (RF). 2008. (In Russ.).
13. Afshari E., Ghambari M., Abdolmalek H. Production of CuSn10 bronze powder from machining chips using jet milling. The International Journal of Advanced Manufacturing Technology. 2017;(92):663–672. https://doi.org/10.1007/s00170-017-0126-3
14. Anas N.S., Ramteke K.N., Kumar R.A., Chouhan R.N., Agnihotri A. Effect of compaction pressure on cold compaction of AA2024 swarf generated during milling operation. Materials Today: Proceedings. 2023. https://doi.org/10.1016/j.matpr.2023.09.106
15. Loginov Yu.N., Zagirov N.N., Ivanov E.V. Evaluation of the level of hardening of aluminum alloy chips intended for subsequent pressure treatment. Obrabotka metallov (tekhnologiya, oborudovanie, instrumenty). 2021;23(1):45–55. (In Russ.). https://doi.org/10.17212/1994-6309-2021-23.1-45-55
16. Dyakonov O.M. Dehydration and deoiling of metal swarf. Litiyo i metallurgiya. 2011;62(3):186–191. (In Russ.).
17. Chang J.I., Lin J.J., Huang J.S., Chang Y.M. Recycling oil and steel from grinding swarf. Resources, Conservation and Recycling. 2006;49:191–201. https://doi.org/10.1016/j.resconrec.2006.03.014
18. Lee Ch.-M., Choi Y.-H., Ha J.-H., Woo W.-S. Eco-friendly technology for recycling of cutting fluids and metal chips: A review. International Journal of Precision Engineering and Manufacturing – green Technology. 2017;4(4):457–468. https://doi.org/10.1007/s40684-017-0051-9
19. Razumov N.G., Masajlo D.V., Sufiyarov V.S., Silin A.O., Popovich A.A., Goncharov I.S. Method of producing powder from metal chips: Patent 2705748 (RF). 2019. (In Russ.).
20. Yaroclavtsev V.M. New concept of metallic cutting waste. Nauka i obrazovanie: nauchnoe izdanie MGTU im. N.E. Baumana. 2013;(2):1–10. (In Russ.). https://doi.org/10.7463/0213.0541318
21. Mendonca P., Dias O., Melo M. Evaluation of high-energy milling efficiency in stainless steel with addition of vanadium carbides. The International Journal of Advanced Manufacturing Technology. 2018;(95):3093–3099. https://doi.org/10.1007/s00170-017-1297-7
22. Mendonca P., Olivera V., Olivera A. Comparison of the effect of carbide addition on particle size reduction on UNS S31803 steel chip millings. The International Journal of Advanced Manufacturing Technology. 2018;(98):1755–1761. https://doi.org/10.1007/s00170-018-2366-2
23. Kopec M., Jóźwiak S., Kowalewski Z. Fe–Al based composite reinforced with ultra-fine Al2O3 oxides for high temperature applications. Journal of Theoretical and Applied Mechanics. 2021;59(3):509–513. https://doi.org/10.15632/jtam-pl/138322
24. Korosteleva E.N., Nikolaev I.O., Korzhova V.V. Features of the structure formation of sintered powder materials using waste metal processing of steel workpieces. Obrabotka metallov (tekhnologiya, oborudovanie, instrumenty) (Metal Working and Material Science). 2022;24(4):192–205. https://doi.org/10.17212/1994-6309-2022-24.4-192-205
25. Korosteleva E.N., Knyazeva A.G., Anisimova M.A., Nikolaev I.O. The impact of impurities on the Al–Fe–C system phase composition changes during sintering. Powder Metallurgy and Functional Coatings. 2023;17(2):5–13. https://doi.org/10.17073/1997-308X-2023-2-5-13
26. Dyakonov O.M. Investigation of physicochemical and mechanical characteristics of steel and cast iron chips. Litiyo i metallurgiya. 2009;(4):161–173. (In Russ.). https://doi.org/10.21122/1683-6065-2009-4-161-173
27. Ryabukhin A.G., Teplyakov Yu.N. Oxidation of iron in air at temperatures of 520–600 ºC (thin films). Vestnik YuUrGU. Seriya: Matematika, fizika, khimiya. 2003;(6): 116–125. (In Russ.).
28. Il’in A.A. Synthesis of iron oxide from metal powders. Izvestiya vuzov. Khimiya i khimicheskaya tekhnologiya. 2019;62(5):62–70. (In Russ.).
29. Rumyantsev R.N., Il’in A.A., Il’in A.P. Mechanochemical synthesis of iron oxide by interaction of metal powders with water. Izvestiya vuzov. Khimiya i khimicheskaya tekhnologiya. 2013;56(6):45–49. (In Russ.).
About the Authors
E. N. KorostelevaRussian Federation
Elena N. Korosteleva – Cand. Sci. (Eng.), Associate Professor at the Department of Mechanical Engineering, School of Advanced Engineering Technologies, National Research Tomsk Polytechnic University; Senior Researcher at the Laboratory of Physics of Powder Material Consolidation, Institute of Strength Physics and Materials Science of the Siberian Branch of the Russian Academy of Sciences (ISPMS SB RAS)
2/4 Akademicheskii Prosp., 634055 Tomsk, Russia
30 Lenina Prosp., Tomsk 634050, Russia
I. O. Nikolaev
Russian Federation
Ivan O. Nikolaev – Research Assistant at the Laboratory of Physics of Powder Material Consolidation
2/4 Akademicheskii Prosp., 634055 Tomsk, Russia
Review
For citations:
Korosteleva E.N., Nikolaev I.O. Evolution of the structural-phase state of steel swarf during its processing into a powdered product. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):6-16. https://doi.org/10.17073/1997-308X-2024-4-6-16