Scroll to:
Synthesis of Co–Mn catalysts for deep oxidation of CO and propane based on natural opoka by low-temperature combustion
https://doi.org/10.17073/1997-308X-2024-6-17-27
Abstract
Natural opoka from the Taskalin deposit in the Republic of Kazakhstan was used as a support for Co–Mn catalysts in the deep oxidation of CO and hydrocarbons. After preliminary preparation of the opoka samples by water washing (opoka I), calcination at 500 °C (opoka II), HCl treatment (opoka III), or combined HCl treatment and calcination at 500 °C (opoka IV), an active phase (AP) consisting of 5 wt. % Co + 5 wt. % Mn (based on metals) was applied via low-temperature combustion of a metal nitrates and urea mixture. The support and catalyst samples were analyzed using XRD and SEM/EDS, and their specific surface area was measured by the BET method. The primary phases identified in the support and catalyst compositions were various modifications of SiO2 , as well as Na-, Ca-, and Mg-aluminosilicates. Due to their low content, AP components in the form of cobalt oxyhydroxide and potassium manganite were detected only on two of the catalyst samples. According to SEM/EDS data, the original nanoscale honeycomb structures on the opoka surface were almost completely destroyed during opoka processing and after AP application. Elemental composition showed notable variability across different granules of both the support and the catalyst, likely due to the natural structural heterogeneity of opoka. It was established that as the complexity of opoka treatment increased, its specific surface area tripled, from 21.0 to 64.1 m2/g. In contrast, the specific surface area of catalysts based on these opoka samples varied irregularly. Testing of the resulting catalysts in the deep oxidation of CO and propane over a temperature range of 150–540 °C revealed substantial activity, with the best performance observed in the catalyst based on water-washed opoka without further treatment. This sample achieved 100 % CO conversion at T = 500 °C and 97 % propane conversion at 540 °C. Thus, natural opoka with minimal processing can serve as an effective support for deep oxidation catalysts for CO and hydrocarbons.
Keywords
For citations:
Jussupkaliyeva R.I., Bystrova I.M., Pomogailo S.I., Borshch V.N. Synthesis of Co–Mn catalysts for deep oxidation of CO and propane based on natural opoka by low-temperature combustion. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):17-27. https://doi.org/10.17073/1997-308X-2024-6-17-27
Introduction
Supported catalysts represent the most widely used class of heterogeneous catalysts. Active phases (AP) are very often deposited on supports made of simple and complex oxides of elements from the main subgroups of the middle of the Periodic Table, such as γ-Al2O3 , SiO2 (typically in hydrated form as silica gel), amorphous and crystalline aluminosilicates, and their complexes with oxides of transition and rare-earth metals. Generally, synthetic materials with a fixed set of physicochemical properties are used. However, there remains significant interest in using natural high-porosity materials as supports due to their low cost and environmental friendliness. The primary challenge usually involves standardizing these natural materials since even within a single deposit, their composition and properties can vary considerably.
The literature documents the use of natural minerals such as bentonite clays (specifically montmorillonite), which are hydroxylated nanoscale-layered aluminosilicates, as acid-base catalysts [1], catalyst supports [2; 3], and photocatalysts [4; 5]. Of particular interest is their dehydrated modification – multilayered aluminosilicate nanotubes known as the natural mineral halloysite [6; 7]. Another popular catalyst support in various processes discussed in the literature is the highly porous silica rock, diatomite [8; 9]. Closely related to diatomite in composition and properties is opoka – a microporous, high-silica sedimentary rock containing up to 92–98 wt. %1 SiO2 . It is widely used in construction as a thermal and sound insulation material, and due to its high adsorption, filtration, and ion-exchange properties, opoka is also employed as an adsorbent and filter filler [10–14]. However, there is no information in the literature regarding its use as a support for AP in catalysis.
Deep catalytic oxidation processes are essential for purifying anthropogenic gas emissions and have long been crucial from an environmental standpoint. Additionally, these processes are applied in flameless heat generators, catalytic burners, fuel cells, and gas composition monitoring systems within fire- and explosion-hazardous industries, among others. According to the literature, catalyst development for these applications is advancing rapidly. It is well established that the most effective catalysts for such reactions are those with AP containing noble metals, effective in both CO oxidation [15; 16] and the deep oxidation of hydrocarbons [17–19].
However, aside from their high cost, a major drawback of noble metal catalysts is their low resistance to catalytic poisons (see, for example, [20]). Consequently, a significant focus in the global literature is on developing and researching new catalytic systems based on non-noble transition and rare-earth elements, particularly in nanoscale forms. Among the most active elements in these systems, Co and Mn are often highlighted, both as supported catalysts on various substrates – such as sialon [21], the previously mentioned diatomite [8], γ-Al2O3 [22], silica gel modified with aluminum oxide [23], foamed silicalite-1 [24], and nanostructured CeO2 [25] – and in monolithic complex oxide forms [26–29].
One of the promising methods for producing supported catalysts is the low-temperature combustion method, also known as self-propagating surface thermosynthesis [22; 23; 30; 31]. This method involves impregnating the support with a solution mixture of oxidizers (usually nitrates of active metals) and a reducer or fuel (a water-soluble organic compound), followed by drying and heating the sample to initiate the combustion reaction. This technique offers several advantages over traditional impregnation methods, including low energy consumption, short reaction time, the formation of highly dispersed (including nanoscale) oxide and/or metallic active phases on the pore surfaces of the support, and the absence of harmful gas emissions (typically only CO2 , nitrogen, and water vapor are released). The low combustion temperature (in our practice, ≤ 360 °C) minimizes the interaction between the AP and the support, preventing particle sintering of the resulting AP.
Previously, using this method, we produced Co-, Mn-, and Ni-containing catalysts on various supports [7; 22; 23], which demonstrated high activity in the deep oxidation of propane and CO.
The aim of this study is to synthesize and examine the physicochemical and catalytic properties of new 5 % Co – 5 % Mn catalyst samples, with the active phase deposited on pre-treated opoka from the Taskalin deposit in Kazakhstan [32] (referred to hereafter as 5Co5Mn/opoka I–IV). These catalysts were evaluated in the deep oxidation of propane and CO.
Before applying the AP, the natural opoka samples underwent several preliminary treatment procedures. A common step for all samples was washing to remove impurities of water-soluble salts and easily washable clay contaminants. The purpose of calcination at 500 °С was to burn off organic impurities in the air and to dehydrate the remaining clay impurities. Treatment with HCl solution was performed to remove oxide impurities, complex oxides, and carbonates containing transition metals, primarily iron, as well as alkaline earth metal carbonates. These procedures were applied to different samples to assess the impact of each treatment step.
Research methodology
Before use, all opoka samples were ground to a particle size of 0.1–0.3 mm, washed several times with distilled water, and dried in an oven at 90 °C. The first part (opoka I) was set aside without further treatment, the second (opoka II) was additionally calcined at 500 °C, the third (opoka III) was washed with a 10 % HCl solution and then rinsed with distilled water, while the fourth (opoka IV) was also washed with a 10 % HCl solution, rinsed with distilled water, and then calcined at 500 °С.
The prepared supports, each weighing 5 g, were impregnated with a solution mixture of metal nitrates (Co(NO3)·6H2O + Mn(NO3)2·6H2O) and urea, with concentration ratios calculated to yield the pure metals. The sample was dried at 90 °C and placed in a flat-bottomed quartz tube reactor, sealed with a dust collection system and purged with argon. The heater at the bottom of the reactor was turned on, and its power remained constant throughout the process. Temperature was monitored by a thermocouple placed in the sample layer at the center of the reactor. After the reaction and cooling, the sample in the argon-filled reactor was stabilized with a 5 % H2O2 solution to prevent spontaneous ignition of any highly dispersed metallic phases in the active phase. The sample was then washed with distilled water and dried at 90 °C. Particles smaller than 0.1 mm were removed from the resulting catalyst. The setup and synthesis procedure were previously described in detail [22; 23].
X-ray diffraction analysis (XRD) of the samples was conducted using a DRON-3M diffractometer (Russia) with FeKα radiation.
The morphology and elemental composition of the catalyst surfaces were examined with a high-resolution field-emission scanning electron microscope, the Zeiss Ultra Plus, based on the Ultra 55 platform (Carl Zeiss, Germany), equipped with an INCA Energy 350 XT microanalysis system from Oxford Instruments. The specific surface area was measured through nitrogen physical adsorption by the BET method on an apparatus designed according to GOST 23401-90.
The obtained samples were tested on a catalytic setup with a flow-through quartz reactor containing a fixed bed of catalyst (1 cm3 of 0.1–0.3 mm fraction). Analysis of the initial gas mixture and reaction products from deep oxidation was conducted using a 5-component gas analyzer, Avtotest 02.03P, with zero-class accuracy (Meta, Russia). The initial gas mixture had the following composition by vol. %: propane – 0.15, CO – 0.6, O2 – 1.6, nitrogen – up to 100 %. The gas flow rate was 120,000 h–1. Experiments were carried out within a temperature range from 150 to 540 °C in 50 °C intervals. CO and propane conversions during deep oxidation were calculated using the formula
\[{X_{R.T}} = \frac{{{C_{R,0}} - {C_{R,T}}}}{{{C_{R,0}}}}100{\rm{ \% }}{\rm{,}}\]
where CR,0 and CR,T are the concentrations of the reactant (CO or propane) in the initial gas mixture and at the reactor outlet at temperature T, respectively, vol. %.
Results and discussion
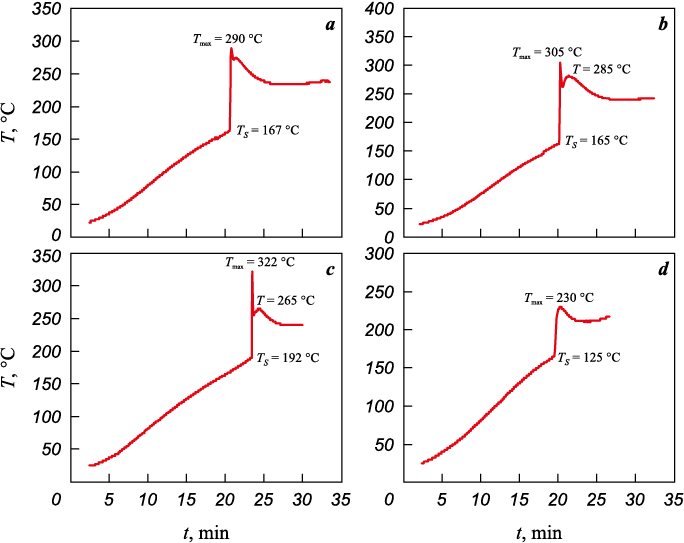
Fig. 1 shows thermograms of the catalyst synthesis process on opoka samples prepared as described above. The self-ignition temperatures (TS ) at the start of the combustion wave and the maximum temperatures (Tmax ) within the combustion wave were recorded. As shown, the TS values for samples on opokas I and II are nearly identical, though the Tmax on opoka II is noticeably higher. The highest self-ignition (192 °C) and combustion (322 °C) temperatures were recorded during synthesis on sample III. Secondary peaks at lower temperatures, likely corresponding to the afterburning wave, were observed on opokas I–III. This phenomenon was also noted in our previous synthesis of a similar AP-based catalyst on γ-Al2O3 [22]. Synthesis on opoka IV proceeded in a low-intensity mode, with minimal TS and Tmax .
Fig. 1. Thermograms of the synthesis process for catalysts 5Co5Mn/opoka I (а), II (b), III (c), and IV (d) |
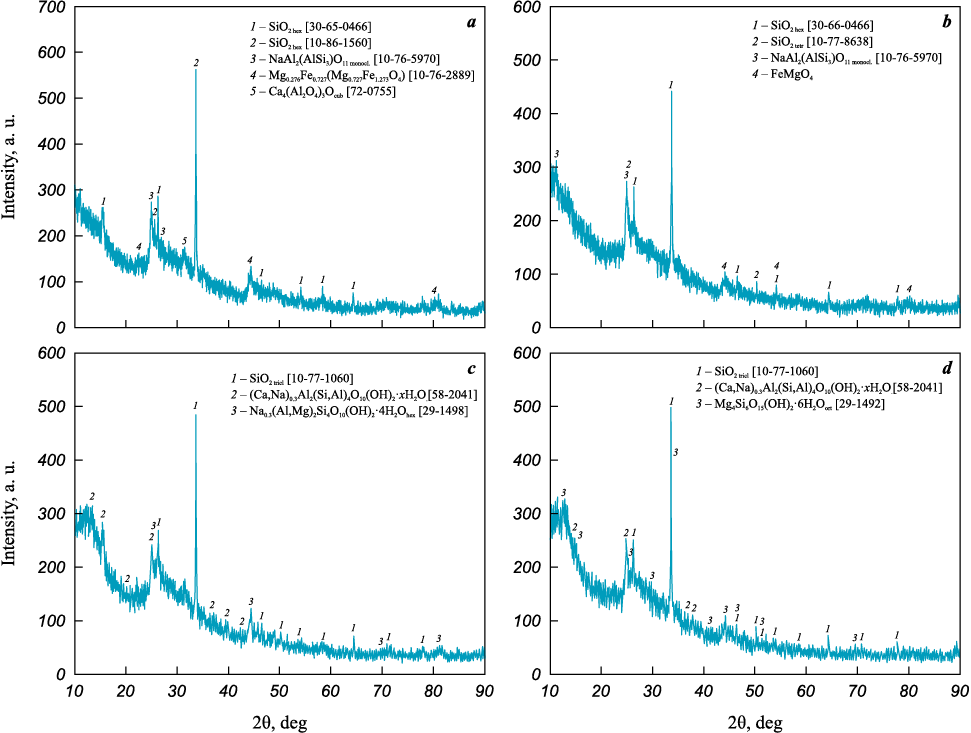
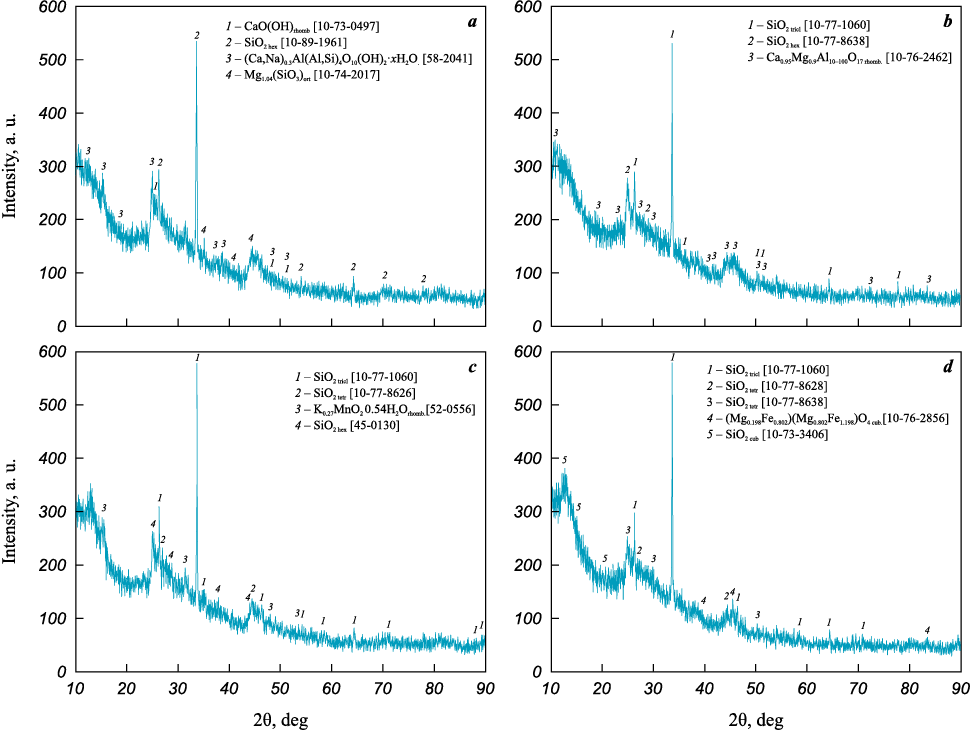
XRD patterns of the prepared supports and their corresponding catalysts are shown in Figs. 2 and 3. Notably, opoka samples I and II contain complex oxide impurity phases of iron and magnesium, which disappear after HCl treatment (see Fig. 2). The primary phases present are various modifications of SiO2 , along with Na-, Ca-, and Mg-aluminosilicates, with a significant portion of amorphous phases also observed.
Fig. 2. XRD patterns of support samples opoka I (а), II (b), III (c), and IV (d)
Fig. 3. XRD patterns of catalysts 5Co5Mn/opoka I (а), II (b), III (c), and IV (d) |
As shown in Fig. 3, Co- and Mn-containing APs appeared only on two catalyst samples – those based on opokas I and III, and separately: in sample I, likely as trivalent Co oxyhydroxide, and in opoka III as potassium manganite. This likely results from the low AP component content, which is near the detection limit of the XRD analysis. Aluminosilicate phases were preserved only in samples based on opokas I and II, while in samples with supports III and IV, the amount of silica phase modifications increased.
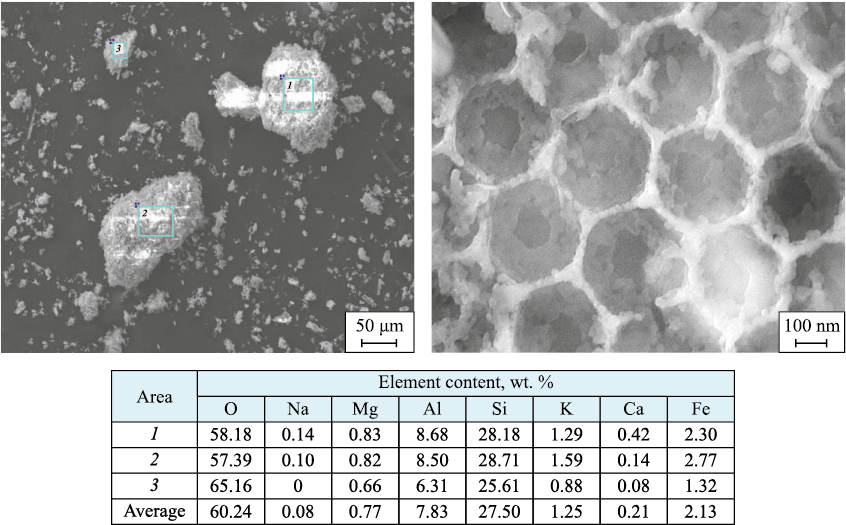
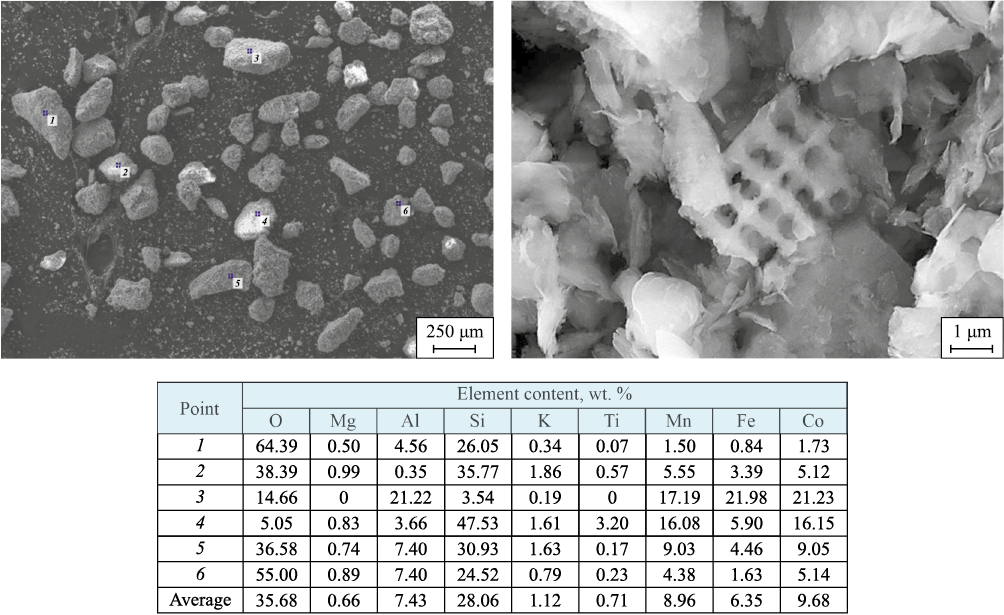
The morphology and surface elemental composition of supports II and IV, as well as the catalyst based on opoka II, were studied using SEM/EDS. The results are shown in Figs. 4–6.
Fig. 4. Surface morphology of opoka II sample (SEM) and elemental composition at marked points
Fig. 5. Surface morphology of opoka IV sample (SEM) and elemental composition at marked points
Fig. 6. Surface morphology of the 5Co5Mn/opoka II catalyst sample (SEM) |
It can be noted that a characteristic feature of the support granule surfaces, both before (Fig. 4) and after (Fig. 5) acid washing, is the presence of nanoscale honeycomb structures with wall thicknesses of ≤ 50 nm, which are clearly visible in Fig. 4. After HCl treatment and calcination, the honeycomb walls appear to melt and thicken noticeably (Fig. 5), though only the application of AP almost completely destroys these formations (Fig. 6). Only isolated elements of the honeycomb structures remain. Notably, as the sample treatment procedures become more complex (opoka I → opoka II → opoka III → opoka IV, followed by AP application), the morphology of the support and catalyst granule surfaces takes on an increasingly fragmented, amorphous appearance.
Elemental microanalysis data show significant variability in the element content across individual granules of both the supports and the catalyst. This applies not only to impurity elements but also to the primary structural elements (Si, Al). This variability may be related to the structural heterogeneity of natural minerals, as noted above. Some samples show detectable amounts of titanium, which does not appear in XRD, likely due to its low concentration, even if present in compound form. Conversely, magnesium, which constitutes a fraction of a percent on the surface, is consistently detected in the form of magnesium silicate on diffractograms, particularly for the opoka IV sample (see Fig. 2, d). The application of AP to the opoka II sample resulted in a noticeable reduction in oxygen content on its surface (compare the data in the Tables in Figs. 4 and 6), even considering that the surface was stabilized with a hydrogen peroxide solution after synthesis. It can be assumed that a portion of weakly bonded oxygen on the support surface reacted during the combustion process when the AP was applied. Significant variations in Co and Mn concentrations on the catalyst granule surfaces are also observed, both in absolute terms and in their relative ratios. This variability likely stems from the structural heterogeneity noted earlier, especially in the number of open pores, where the AP precursor solution penetrates through capillary action prior to synthesis.
The specific surface area (Ssp ) of certain support and catalyst samples, determined by the BET method, is shown below, in m2/g:
Opoka I . . . . . . . . . . . . . . 21.0
Opoka II . . . . . . . . . . . . . . 42.0
Opoka IV . . . . . . . . . . . . . . 64.1
5Co5Mn/opoka I . . . . . . . . 40.6
5Co5Mn/opoka II . . . . . . . 29.5
5Co5Mn/opoka IV . . . . . . . 62.8
A distinctive feature of the support treatment process is evident: as the treatment becomes more complex, the specific surface area of the samples increases, likely due to the exposure of an increasing number of fine pores. However, the application of the AP has varying effects on this value. As can be seen, the catalyst based on opoka I has twice the specific surface area Ssp of the initial support. Conversely, for the catalyst based on opoka II, this trend is reversed, while for the 5Co5Mn/opoka IV sample, there was almost no change. This may indicate differences in the dispersion of the AP formed during synthesis, depending on the surface morphology and, to some extent, the elemental composition of the different support samples.
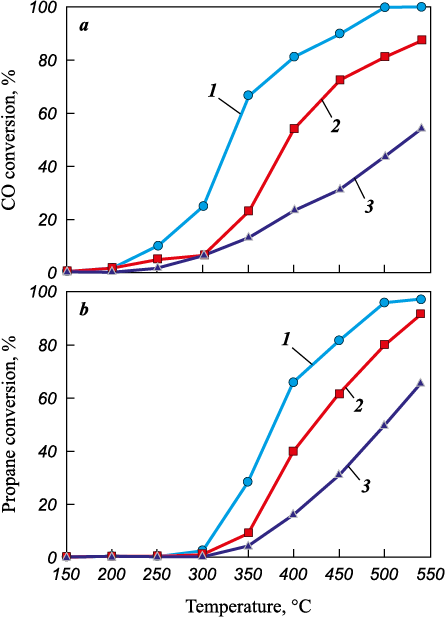
A series of catalysts based on treated opoka was tested in the deep oxidation of propane and CO. The results are shown in Fig. 7. According to these data, the opoka-based catalysts demonstrated fairly high activity in both CO oxidation and propane deep oxidation. In the first case, 100 % CO conversion was achieved at 500 °C on the 5Co5Mn/opoka I catalyst (Fig. 7, a), with its activity being higher across the entire temperature range. In the second case, this same catalyst achieved a propane conversion of 97 % at 540 °C (Fig. 7, b). The sample based on opoka IV showed the lowest activity in these processes, despite having the highest specific surface area among the samples. It should be noted that for the 5Co5Mn/opoka II and 5Co5Mn/opoka IV samples, propane conversion in the high-temperature region (≥ 500 °C) exceeded CO conversion.
Fig. 7. Temperature dependences of CO (a) and propane (b) conversions on catalyst samples |
Conclusion
For the first time, catalysts with a CoMn active phase (5 % + 5 % based on metals) on differently treated opoka were obtained using the low-temperature combustion method with mixtures of Co and Mn nitrates and urea. XRD analysis showed that, following acid treatment and subsequent calcination, iron-magnesium complex oxide impurities are removed from the opoka samples, with the main phases being various modifications of silica, along with Na-, Ca-, and Mg-aluminosilicates, and a significant proportion of amorphous phases.
SEM/EDS analysis of the morphology and surface elemental composition of the supports and catalyst revealed degradation of the initial nanoscale honeycomb structures as opoka processing became more complex, with further degradation upon AP application. A significant variability in elemental content among individual granules of both supports and catalysts was observed, likely due to the structural heterogeneity of this natural mineral.
The specific surface area of the support samples increased with more complex processing steps, but the surface area of the catalysts varied non-monotonically. Testing of several catalysts showed the activity order in the deep oxidation of both CO and propane as follows: 5Co5Mn/opoka I > 5Co5Mn/opoka II > 5Co5Mn/opoka IV, with CO conversion reaching 100 % at 500 °C and propane conversion reaching 97 % at 540 °C on the 5Co5Mn/opoka I sample. The sample based on opoka IV demonstrated the lowest activity in these processes, despite having the highest specific surface area among the catalysts studied (62.8 m2/g).
Thus, natural opoka with minimal processing can serve as an effective support for deep oxidation catalysts for hydrocarbons and CO.
References
1. Gandhi D., Bandyopadhyay R., Soni B. Naturally occurring bentonite clay: Structural augmentation, characterization and application as catalyst. Materials Today: Proceedings. 2022;57(1):194–201. https://doi.org/10.1016/j.matpr.2022.02.346
2. Borah D., Nath H., Saikia H. Modification of bentonite clay & its applications: A review. Reviews in Inorganic Chemistry. 2022;42(3):265–282. https://doi.org/10.1515/revic-2021-0030
3. Vaculíková L.,Valovičová V., Plevová E., Napruszewska B.D., Duraczyńska D., Karcz R., Serwicka E.M. Synthesis, characterization and catalytic activity of cryptomelane/montmorillonite composites. Applied Clay Science. 2021;202(1):105977. https://doi.org/10.1016/j.clay.2021.105977
4. Zhou D., Jiang D., Jing H., Yin C., Li C. Natural aluminosilicate nanoclay mineral for photocatalytic applications: Influence of the surface properties in photocatalysis. Applied Clay Science. 2024;249(1):107240. https://doi.org/10.1016/j.clay.2023.107240
5. Feng J., Hu X., Yue P.L. Novel bentonite clay-based Fe-nanocomposite as a heterogeneous catalyst for photo-Fenton discoloration and mineralization of orange II. Environmental Science & Technology. 2004;38(1):269–275. https://doi.org/10.1021/es034515c
6. Massaro M., Noto R., Riela S. Halloysite nanotubes: Smart nanomaterials in catalysis. Catalysts. 2022;12(2):149. https://doi.org/10.3390/catal12020149
7. Borshch V.N., Bystrova I.M., Pugacheva E.V., Smirnova E.M., Stavitskaya A.V., Vinokurov V.A. Low-temperature combustion synthesis of halloysite-based catalysts for the deep oxidation of hydrocarbons and carbon monoxide and the methanation of carbon dioxide. Kinetics and Catalysis. 2022;63(6):775–786. https://doi.org/10.1134/S0023158422060027
8. Liu Q., Li M.., Wang S., Lv S., Han F., Xi Y., Cao Z., Ouyang J. Ultrathin 3D CoMn nanoflowers coupled diatomite for highly efficient catalytic oxidation of CO and propane. Chemical Engineering Journal. 2023;477:147102. https://doi.org/10.1016/j.cej.2023.147102
9. Liang L., Miao C., Ke X., Peng Y., Chen G., Ouyang J. A superior strategy for CO2 methanation under atmospheric pressure: Organic acid-assisted Co nanoparticles assembly on diatomite. Fuel. 2023;351:128931. https://doi.org/10.1016/j.fuel.2023.128931
10. Mal’kova V.N. Method for obtaining a sorbent for cleaning solid surfaces and water from oil and liquid petroleum products: Patent 2642799. (RF). 2018. (In Russ.).
11. Smol M., Włóka D. Use of natural sorbents in the processes of removing biogenic compounds from the aquatic environment. Sustainability. 2022;14:6432. https://doi.org/10.3390/su14116432
12. Kotlyar V.D., Bratskiy D.I., Ustinov A.V. Material composition and pre-firing ceramic properties of clay flasks. Inzhenernyi vestnik Dona. 2010;4:47–59. (In Russ.). http://www.ivdon.ru/magazine/archive/n4y2010/249
13. Makarov D.V., Manakova N.K., Suvorova O.V. Production of rock-based foam-glass materials. Glass and Ceramics. 2023;79(9):411–417. https://doi.org/10.1007/s10717-023-00522-8
14. Kurmangazhy G., Tazhibayeva S., Musabekov K., Sydykbayeva S., Zhakipbaev B. Magnetite-gaize composite stabilized with polyacrylic acid. Chemical Bulletin of Kazakh National University. 2020;98(3):12–17. https://doi.org/10.15328/cb1160
15. Schilling C., Ziemba M., Hess C., Ganduglia-Pirovano M.V. Identification of single-atom active sites in CO oxidation over oxide-supported Au catalysts. Journal of Catalysis. 2020;383:264–272. https://doi.org/10.1016/j.jcat.2020.01.022
16. Fan J., Hu S., Li C., Wang Y., Chen G. Effect of loading method on catalytic performance of Pt/CeO2 system for CO oxidation. Molecular Catalysis. 2024;558:114013. https://doi.org/10.1016/j.mcat.2024.114013
17. Auvray X., Lindholm A., Milh M., Olsson L. The addition of alkali and alkaline earth metals to Pd/Al2O3 to promote methane combustion. Effect of Pd and Ca loading. Catalysis Today. 2018;299:212–218. https://doi.org/10.1016/j.cattod.2017.05.066
18. Zhang W-X., Zhao X., Xu L-Y., Xia S., Zhou Y-F., Chen C-L., He H-H., Luo M-F., Chen J. Unveiling the crucial active sites responsible for CO, n-heptane, and toluene oxidation over Pt/ZrO2 catalyst. Molecular Catalysis. 2024;558:114015. https://doi.org/10.1016/j.mcat.2024.114015
19. Shikina N.V., Yashnik S.A., Gavrilova A.A., Nikolaeva O.A., Dovlitova L.S., Ishchenko A.V., Ismagilov Z.R. Effect of the conditions of solution combustion synthesis on the properties of monolithic Pt–MnOx catalysts for deep oxidation of hydrocarbons. Kinetics and Catalysis. 2020;61(5):809–823. https://doi.org/10.1134/S0023158420050110
20. Montenegro N.D., Epling W.S. Effects of SO2 poisoning and regeneration on spinel containing CH4 oxidation catalysts. Applied Catalysis B: Environmental. 2023;336: 122894. https://doi.org/10.1016/j.apcatb.2023.122894
21. Borshch V.N., Zhuk S.Ya., Vakin N.A., Smirnov K.L., Borovinskaya I.P., Merzhanov A.G. Sialons as a new class of supports for oxidation catalysts. Doklady Physical Chemistry. 2008;420(2)121–124. https://doi.org/10.1016/10.1134/S0012501608060018
22. Borshch V.N., Dement’eva I.M., Khomenko N.Yu. Supported polymetallic catalysts by self-propagating surface synthesis. International Journal of Self-Propagating High-Temperature Synthesis. 2019;28(1):45–49. https://doi.org/10.3103/S1061386219010059
23. Borshch V.N., Bystrova I.M., Boyarchenko, O.D., Khomenko N.Yu., Belousova O.V. Low-temperature combustion synthesis and characterization of Co-containing catalysts based on modified silica gel. International Journal of Self-Propagating High-Temperature Synthesis. 2023;32(2):126–138. https://doi.org/10.3103/S1061386223020024
24. Guan Y., Shen H., Guo X., Mao B., Yang Z., Zhou Y., Liang H., Fan X., Jiao Y., Zhang J. Structured hierarchical Mn–Co mixed oxides supported on silicalite-1 foam catalyst for catalytic combustion. Chinese Journal of Chemical Engineering. 2020;28:2319–2327. https://doi.org/10.1016/j.cjche.2020.06.019
25. Liu Z., Li J., Wang R. CeO2 nanorods supported M–Co bimetallic oxides (M = Fe, Ni, Cu) for catalytic CO and C3H8 oxidation. Journal of Colloid and Interface Science. 2020;560:91–102. https://doi.org/10.1016/j.jcis.2019.10.046
26. Zhang X., Ye J., Yuan J., Cai T., Xiao B., Liu Z., Zhao K., Yang L., He D. Excellent low-temperature catalytic performance of nanosheet Co–Mn oxides for total benzene oxidation. Applied Catalysis A: General. 2018;566:104–112. https://doi.org/10.1016/j.apcata.2018.05.039
27. Feng C., Chen C., Xiong G., Yang D., Wang Z., Pan Y., Fei Z., Lu Y., Liu Y., Zhang R., Li X. Cr-doping regulates Mn3O4 spinel structure for efficient total oxidation of propane: Structural effects and reaction mechanism determination. Applied Catalysis B: Environmental. 2023;328:122528. https://doi.org/10.1016/j.apcatb.2023.122528
28. Shen K., Wang C.-Y., Rai R.K., Stach E.A., Vohs J.M., Gorte R.J. Synthesis of thin-film CuMn2O4 for low-temperature CO oxidation. Applied Catalysis A: General. 2024;682:119823. https://doi.org/10.1016/j.apcata.2024.119823
29. González-Cobos J., Mylonoyannis B., Chai G., Zhang W., Tian C., Kaddouri A., Gil S. Low-temperature gas-phase toluene catalytic combustion over modified CoCr2O4 spinel catalysts: Effect of Co/Cr content and calcination temperature. Applied Catalysis A: General. 2023;657:119162. https://doi.org/10.1016/j.apcata.2023.119162
30. Zav’yalova U.F., Tret’yakov V.F., Burdeinaya T.N., Lunin V.V., Shitova N.B., Ryzhova N.D., Shmakov A.N., Nizovskii A.I., Tsyrul’nikov P.G. Self-propagating synthesis of supported oxide catalysts for deep oxidation of CO and hydrocarbons. Kinetics and Catalysis. 2005; 46(5):752–757. https://doi.org/10.1007/s10975-005-0132-6
31. Kotolevich Y.S., Mamontov G.V., Vodyankina O.V., Petrova N.I., Smirnova N.S., Tsyryul’nikov P.G., Trenikhin M.V., Nizovskii A.I., Kalinkin A.V., Smirnov M.Y., Goncharov V.B. Catalytic Pd–Ag nanoparticles immobilized on fiber glass by surface self-propagating thermal synthesis. International Journal of Self-Propagating High-Temperature Synthesis. 2017;26(4):234–239. https://doi.org/10.3103/S1061386217040045
32. Smirnov P.V., Zhakipbayev B.E., Staroselets D.A., Deryagina O.I., Batalin G.A., Gareev B.I., Vergunov A.V. Diatomites and opoka from Western Kazakhstan deposits: lithogeochemistry, structural and textural parameters, potential of use. Izvestiya Tomskogo politekhnicheskogo universiteta. Inzhiniring georesursov. 2023;334(7)187–201. (In Russ.). https://doi.org/10.18799/24131830/2023/7/4046
About the Authors
R. I. JussupkaliyevaKazakhstan
Roza I. Jussupkaliyeva – Master Sci. (Tech.), Senior Lecturer
51 Zhangir Khan Str., Uralsk 090009, Republic of Kazakhstan
I. M. Bystrova
Russian Federation
Inna M. Bystrova – Junior Researcher, Laboratory of Catalytic Processes
8 Academician Osip’yan Str., Chernogolovka, Moscow Region 142432, Russia
S. I. Pomogailo
Russian Federation
Svetlana I. Pomogailo – Cand. Sci. (Chem.), Senior Research Scientist, Laboratory of Catalytic Processes
8 Academician Osip’yan Str., Chernogolovka, Moscow Region 142432, Russia
V. N. Borshch
Russian Federation
Vyacheslav N. Borshch – Cand. Sci. (Chem.), Leading Researcher, Laboratory of Catalytic Processes
8 Academician Osip’yan Str., Chernogolovka, Moscow Region 142432, Russia
Review
For citations:
Jussupkaliyeva R.I., Bystrova I.M., Pomogailo S.I., Borshch V.N. Synthesis of Co–Mn catalysts for deep oxidation of CO and propane based on natural opoka by low-temperature combustion. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):17-27. https://doi.org/10.17073/1997-308X-2024-6-17-27