Scroll to:
Azide self-propagating high-temperature synthesis of a highly dispersed AlN–SiC powder composition using polytetrafluoroethylene
https://doi.org/10.17073/1997-308X-2024-6-28-43
Abstract
Composite ceramics from aluminum nitride with silicon carbide (AlN–SiC) is promising for applications in both metallurgy and mechanical engineering as a refractory and structural material with enhanced properties, as well as in electronics and photonics as an advanced material for creating various high-performance devices. To fabricate products with optimal properties, compositions (mixtures) of highly dispersed AlN and SiC powders with particle sizes of less than 1 μm must be used. This study is dedicated to improving a simple, energy-efficient method of azide self-propagating high-temperature synthesis (SHS) for such powder compositions, using mixtures of sodium azide (NaN3 ) powder and elemental powders of aluminum, silicon, and carbon with the addition of polytetrafluoroethylene (PTFE) powder as an activating and carbidizing additive. During the combustion of these mixtures in a bulk or pressed form in a reactor under 3 MPa of nitrogen gas pressure, the temperature, pressure, and yield of solid combustion products were evaluated. Scanning electron microscopy and X-ray phase analysis were employed to determine the microstructure and phase composition of the combustion products. The addition of PTFE helped to eliminate, in most cases, the drawbacks of the traditional azide SHS approach using halide salts such as (NH4)2SiF6 , AlF3 , and NH4F. While maintaining the high dispersity of the synthesized AlN–SiC powder compositions, their phase composition, particularly in pressed charges, became significantly closer to the targeted theoretical composition, with a substantial increase in SiC phase content and the elimination of undesirable by-products such as silicon nitride and the water-insoluble cryolite salt Na3AlF6 .
Keywords
For citations:
Amosov A.P., Titova Yu.V., Uvarova I.A., Belova G.S. Azide self-propagating high-temperature synthesis of a highly dispersed AlN–SiC powder composition using polytetrafluoroethylene. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):28-43. https://doi.org/10.17073/1997-308X-2024-6-28-43
Introduction
Aluminum nitride (AlN) is one of the foremost materials in technical ceramics [1]. It exhibits an exceptional combination of physical, electrical, and chemical properties: low density, high melting (or decomposition) temperature, thermal conductivity, electrical resistance, hardness, strength, wear resistance, and heat resistance, as well as resistance to thermal shock, acids, and molten metals, and stability at elevated temperatures in various gaseous environments. Owing to these characteristics, aluminum nitride has long found applications across diverse industrial sectors, particularly for high-temperature applications. It is widely used as a refractory material for lining tanks, electrolyzers, and containers in metallurgy and chemical engineering, as well as for producing protective sheaths for metallic thermocouples and manufacturing crucibles. AlN ceramics are among the most widely used electrical insulating materials. Furthermore, aluminum nitride is employed as a structural material for parts operating in aggressive environments and in cutting tools [2]. Intensive research to enhance its physical and mechanical properties continues [3].
However, due to the unique physical properties of AlN, its application in electronics and photonics has recently expanded rapidly [4; 5]. In electronics, this expansion is driven by its excellent heat dissipation capabilities in electronic devices, attributed to its uniquely high thermal conductivity combined with high electrical resistance and a coefficient of thermal expansion (CTE) close to that of silicon. This has led to a transition to aluminum nitride in nearly all areas of electronic component production, where the highly toxic beryllium oxide was traditionally used [4]. In photonics, AlN’s wide bandgap, broad transparency window (covering the range from ultraviolet to mid-infrared), and significant second-order nonlinear optical effect have further broadened its utility. Additionally, AlN exhibits piezoelectric and pyroelectric effects, enabling its use in opto-mechanical devices and pyroelectric photodetectors, respectively [5]. However, in both these fields and in high-temperature structural applications, the use of aluminum nitride is constrained by its brittleness, specifically its relatively low fracture toughness and thermal shock resistance [6].
In this regard, significant attention is being drawn to the development of composite ceramics of aluminum nitride with silicon carbide (SiC), which, in addition to its high thermal conductivity and heat resistance, possesses substantially improved mechanical properties (hardness, fracture toughness, thermal stability, and creep resistance) [7; 8]. Silicon carbide is also attractive due to its crystal structure, which is similar to that of aluminum nitride, allowing it to form a single-phase homogeneous solid solution with AlN, enhancing flexural strength and fracture toughness, thereby reducing brittleness [9; 10]. Additionally, in a two-phase state with sintered, spatially separated powder components of AlN and SiC, AlN–SiC composites exhibit significantly better toughness and thermal stability [8; 11]. By controlling the grain size of AlN and SiC, it is possible to obtain AlN–SiC material with high thermal conductivity [12]. Notably, reducing grain size is another important approach to improving nearly all properties of AlN ceramics and AlN–SiC ceramic composites as a whole [13; 14].
AlN–SiC composite ceramics have shown promise not only for applications in metallurgy and mechanical engineering as a refractory and structural material with enhanced properties but also, as noted above, in electronics and photonics for the creation of a variety of high-performance devices [4; 5; 15].
Traditional energy-intensive methods for producing AlN–SiC ceramics include pressureless sintering of SiC and AlN ceramic powders, hot pressing, and injection molding, all of which require temperatures of around 2000 °C and prolonged holding times of several hours [16–19]. More modern and less energy-intensive methods include spark plasma sintering, microwave heating, and additive 3D-printing technologies; however, these require costly equipment [20–22].
In both methods, the starting material must be a composition (mixture) of AlN and SiC powders. To achieve the best properties of the resulting AlN–SiC ceramics, these powders should be as fine as possible: highly dispersed (submicron) with particle sizes d < 1 μm or even nanoscale with d < 100 μm (0.1 μm) [13; 23]. There are two approaches to producing AlN and SiC powder mixtures: ex-situ and in-situ. The first approach is the simplest and most common for producing composite ceramics and involves mixing ready-made AlN and SiC powders, compacting, and sintering them. However, for highly dispersed powders, especially nanopowders, two issues arise: high cost and the near impossibility of achieving uniform mechanical mixing due to the strong tendency of nanoparticles to form durable agglomerates that are challenging to break up during mixing. The in-situ processes for producing composite ceramics involve the chemical synthesis of AlN and SiC powder particles within the composite from a mixture of significantly cheaper starting reagents, allowing for better dispersion of the synthesized particles. These technologies are thus more favorable for obtaining mixtures of highly dispersed and nanoscale AlN and SiC powders [13; 23].
Traditional in-situ methods for producing mixtures of AlN and SiC powder components, or their solid solutions (furnace method, plasma-chemical synthesis, carbothermal synthesis, gas-phase deposition, etc.), are known for their high energy consumption, complex equipment requirements, and inconsistent ability to yield nanoscale powders and nanopowder compositions [10; 13; 14; 16; 24–26].
Self-propagating high-temperature synthesis (SHS), or “combustion synthesis”, presents distinct advantages over conventional technologies [16; 27–29]. The in-situ SHS approach is notably more cost-effective, as the AlN–SiC composite synthesis is driven by self-sustained combustion heat, requiring only simple equipment and low-cost precursor reagents, such as Al, Si, C (carbon black), Si3N4 powders, and gaseous N2 . This method has therefore garnered significant research interest, with various powder mixtures being examined for their combustion efficiency in producing AlN–SiC composites [27; 30–33]. A review of these studies [34] reveals that, in all cases, the synthesized AlN–SiC ceramics exhibit micron-sized particle structures.
To achieve a composition of highly dispersed (d < 1 μm) AlN–SiC powders, the authors investigated a variant of synthesis known as azide SHS technology, where sodium azide (NaN3) powder serves as the nitriding agent. Additionally, various activating halide salts are introduced alongside elemental reagents, promoting relatively low combustion temperatures, the formation of significant intermediate vapor-gas reaction products, and the generation of condensed and gaseous by-products that effectively separate target powder particles, thus preventing their agglomeration into larger particles [34–37]. The main findings of these investigations are summarized as follows.
Azide SHS technology was employed to synthesize AlN–SiC powder compositions at five molar ratios of the target phases aluminum nitride and silicon carbide AlN:SiC = 4:1, 2:1, 1:1, 1:2, and 1:4, according to stoichiometric equations involving the use of halide salts (NH4 )2SiF6 , AlF3 and NH4F [37].
xSi–yAl–NaN3–(NH4)2SiF6–(x + 1)C system
| Si + 8Al + 6NaN3 + (NH4)2SiF6 + 2C = 8AlN + 2SiC + 6NaF + 4H2 + 6N2 , | (1) |
| Si + 4Al + 6NaN3 + (NH4)2SiF6 + 2C = 4AlN + 2SiC + 6NaF + 4H2 + 8N2 , | (2) |
| Si + 2Al + 6NaN3 + (NH4)2SiF6 + 2C = 2AlN + 2SiC + 6NaF + 4H2 + 9N2 , | (3) |
| 3Si + 2Al + 6NaN3 + (NH4)2SiF6 + 4C = 2AlN + 4SiC + 6NaF + 4H2 +9N2 , | (4) |
| 7Si + 2Al + 6NaN3 + (NH4)2SiF6 + 8C = 2AlN + 8SiC + 6NaF + 4H2 +9N2 . | (5) |
xSi–yAl–NaN3–AlF3xC system
| 2Si + 7Al + 3NaN3 + AlF3 + 2C =8AlN + 2SiC + 3NaF + 0.5N2 , | (6) |
| 2Si + 3Al + 3NaN3 + AlF3 + 2C =4AlN + 2SiC + 3NaF + 2.5N2 , | (7) |
| 2Si + Al + 3NaN3 + AlF3 + 2C =2AlN + 2SiC + 3NaF + 3.5N2 , | (8) |
| 4Si + Al + 3NaN3 + AlF3 + 4C =2AlN + 4SiC+ 3NaF + 3.5N2 , | (9) |
| 8Si + Al + 3NaN3 + AlF3 + 8C =2AlN + 8SiC + 3NaF + 3.5N2 . | (10) |
xSi–yAl–NaN3–NH4FxC system
| Si + 4Al + NaN3 + NH4F + C =4AlN + SiC + NaF + 2H2 , | (11) |
| Si + 2Al + NaN3 + NH4F + C =2AlN + SiC + NaF + 2H2 + N2 , | (12) |
| Si + Al + NaN3 + NH4F + C =AlN + SiC + NaF + 2H2 + 1.5N2 , | (13) |
| 2Si + Al + NaN3 + NH4F + 2C =AlN + 2SiC + NaF + 2H2 + 1.5N2 , | (14) |
| 4Si + Al + NaN3 + NH4F + 4C =AlN + 4SiC + NaF + 2H2 + 1.5N2 . | (15) |
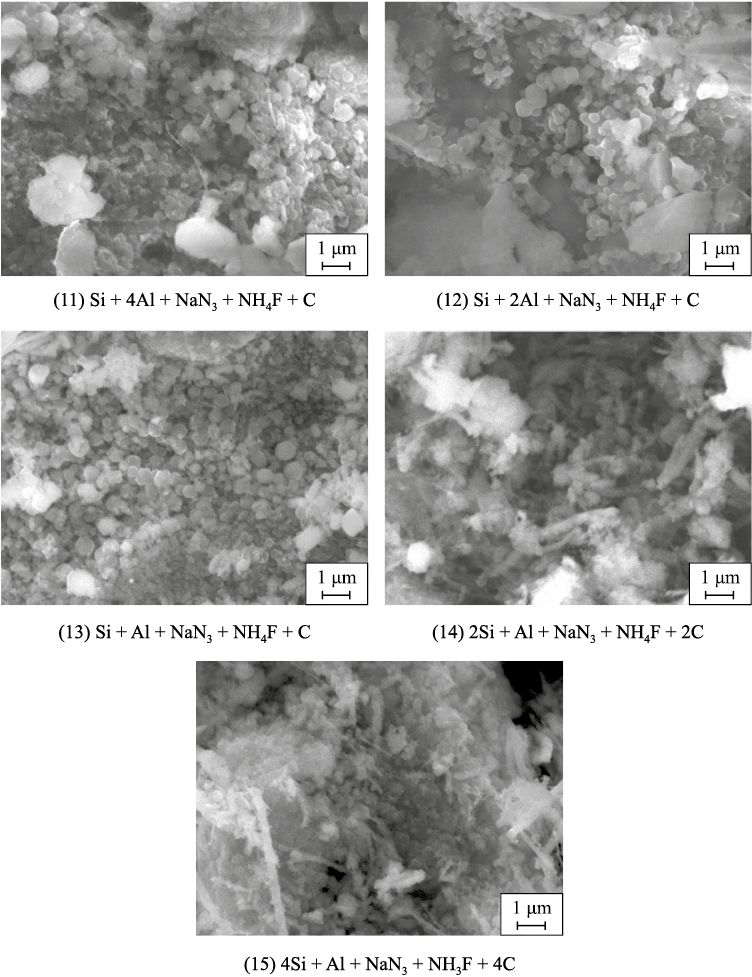
The initial reagent mixtures (charges) from equations (1)–(15) were burned in bulk form with a relative density of 0.4 in a tracing paper cup with a diameter of 30 mm and a height of 45 mm inside a 4.5 L azide SHS reactor at a nitrogen gas pressure of 4 MPa. The cooled combustion product was removed from the reactor, crushed to a loose powdered state in a porcelain mortar, and washed with water to remove the by-product sodium fluoride (NaF). In most cases, the combustion product consisted of a highly dispersed powder of complex composition, appearing as equiaxed submicron particles with sizes ranging from 100 nm to 1 μm and fibers with diameters of 50–500 nm and lengths up to 5 μm, with a tendency toward an increased proportion of finer particles as the SiC content in the AlN–SiC composition increased. This is illustrated in Fig. 1 for the system with the halide salt NH4F, and in study [34] for the system with (NH4)2SiF6 . The results comparing the phase composition of the washed solid combustion products from charges (1)–(15) (determined experimentally) with the theoretical composition of the target phases expected according to the stoichiometric equations (1)–(15) are presented in Table 1. As shown, the experimental phase composition of the azide SHS products significantly deviates from the expected theoretical composition of the AlN–SiC powder compositions. First, the content of the target phases AlN and SiC is lower than theoretically expected, particularly the SiC phase, whose quantity is on average half of the theoretical amount. Second, an undesirable side phase of silicon nitride in α- and β-modifications is present in considerable amounts (up to 22.1 and 15.2 wt. %, respectively). Third, there is a noticeable amount (from 4.1 to 9.8 wt. %) of an unwanted water-insoluble impurity, the cryolite salt Na3AlF6 .
Fig. 1. SEM images of combustion products of charges with NH4F according to equations (11)–(15)
Table 1. Theoretical and experimental phase composition
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In this context, the aim of this study was to bring the experimental composition of the target highly dispersed AlN and SiC powder mixture closer to the theoretical composition according to the stoichiometric equations of azide SHS Technology by adjusting the composition of the initial reagents and the conditions of the azide SHS process. Primarily, it is essential to significantly increase the content of the SiC carbide phase in the azide SHS product. Several approaches are known [38] to promote the formation of SiC during the combustion of a silicon and carbon black powder mixture via the weakly exothermic reaction
| Si + C → SiC: | (16) |
– preheating the charge;
– applying an electric field;
– mechanical activation of the charge;
– conducting combustion in a gaseous nitrogen or air atmosphere;
– chemical activation with catalytic powder additives.
Most of these approaches can lead to submicron or even nanoscale SiC powders in the combustion mode. In this study, the simplest approach was chosen – using the most effective catalytic additive, powdered polytetrafluoroethylene (PTFE) [39; 40].
In the first study on this topic, the chemical transformation mechanism and combustion modes in the silicon–carbon–PTFE system were investigated, depending on the ratios of the starting components, the pressure of the inert atmosphere, ignition source temperature, and sample diameter [39]. Subsequently, combustion in this system was studied in a nitrogen atmosphere at varying pressures to produce SHS composite ceramic powders of Si3N4–SiC and silicon carbide [41]. It was found that without PTFE, the synthesized Si3N4–SiC composites contained between 5 and 60 wt. % SiC, whereas the use of an activating additive at 5–15 wt. % increased the SiC content to 100 %. Study [42] demonstrated that partially replacing carbon with PTFE led to complete carbidizing in the reaction Si + 0.9C + 0.05C2F4 = SiC + 0.1F2 in a nitrogen atmosphere at 3 MPa, producing SiC particles with an average size of around 200 nm. Study [43] showed that PTFE can be used not only as an activating additive but also as a carbidizing reagent when fully replacing technical carbon during combustion of a bulk Si + PTFE mixture in gaseous argon at 0.5 MPa, resulting in the synthesis of silicon carbide in the form of fibers with diameters of 100–500 nm and equiaxed particles of 0.5–3.0 μm, which aggregate into clusters. However, the yield of SiC synthesis was very low: only about 10 % of the total charge mass reacted to form fibrous SiC, with the remainder consisting of unreacted carbon and silicon [43].
The reactions for silicon carbide formation involving polytetrafluoroethylene (–C2F4–)n can be represented as follows [43; 44]:
| 2(–C2F4–)n → CF4(g) + 2CF2(g) + C(s), | (17) |
| 4Si(l) + 2CF4(g) + 2CF2(g) → SiF4(g) + 2SiF3(g) + SiF2(g) + 4C(s), | (18) |
| 2SiF2(g) + 2SiF3(g) → 2.5SiF4(g) + 1.5Si(l), | (19) |
| Si(l) + C(s) → SiC(s). | (20) |
The first stage (17) involves the exothermic decomposition of PTFE in the preheating zone into gaseous fluorides and solid carbon particles. The intermediate stages (18) and (19) represent interactions between the gaseous fluorides with each other and with molten silicon particles, while the final stage (20) depicts the reaction between silicon and carbon particles (both the original carbon in the form of carbon black and the carbon generated from PTFE decomposition) to form the target silicon carbide. The completion of all stages results in the formation of highly dispersed SiC, whereas only the first stage yields gaseous fluorides and carbon black particles [43]. Combustion involving PTFE occurs at a high rate with intense gas release, which may cause dispersion of the charge components, preventing the silicon and carbon particles from reacting with each other, a reaction that is unlikely to proceed in the gas phase [43]. Therefore, a PTFE-containing charge should be in a pressed, rather than bulk, form, as a briquette with a diameter of at least 30 mm, and combusted under excess gas pressure in the SHS reactor to prevent PTFE decomposition products from escaping the reaction zone [39; 42–44].
In the present study, these findings were used to increase the SiC phase content in the target composition of highly dispersed AlN–SiC powders produced via azide SHS technology with PTFE, aiming to bring the experimental composition of the target highly dispersed AlN and SiC powders closer to the theoretical composition in accordance with the stoichiometric equations of azide SHS.
Research methodology
In the study of AlN–SiC composition synthesis processes using azide SHS technology with PTFE, the following initial charge components were used (here and throughout: wt. %):
– silicon powder, grade Kr00 (main substance content ≥ 99.9 %, average particle size d = 40 μm);
– aluminum powder, grade PA-4 (≥ 98.0 %, d = 100 μm);
– sodium azide powder, analytical grade (≥ 98.71 %, d = 100 μm);
– polytetrafluoroethylene (PTFE), grade PN-40 (≥ 99.0 %, d = 40 μm);
– technical carbon black, grade P701 (≥ 88.0 %, d = 70 nm, in the form of agglomerates up to 1 μm).
PTFE was used as an activating and carbidizing additive, partially replacing technical carbon black. Based on the findings from studies [39; 41; 42], a carbidizing mixture of technical carbon and PTFE of various compositions was used to carbidize silicon, equivalent to 1 mole of carbidizing carbon:
| 0.9C + 0.05C2F4 , | (A) |
| 0.8C + 0.1C2F4 , | (B) |
| 0.7C + 0.15C2F4 . | (C) |
These mixtures correspond to PTFE concentrations of 5 to 15 %, necessary for obtaining pure SiC through reaction (16) and achieving an increased SiC content in composites with silicon nitride [41]. Sodium azide (NaN3) was added to the charge in an amount sufficient to neutralize the fluorine released during the complete decomposition of PTFE, binding it into the water-soluble compound NaF, which can be easily removed from the azide SHS product by washing with water. As a result, the stoichiometric equations of the azide SHS for AlN–SiC powder compositions with 5 molar ratios of target phases AlN:SiC = 4:1, 2:1, 1:1, 1:2 and 1:4 using carbidizing mixture (A) with PTFE under combustion in gaseous nitrogen are as follows:
| 4Al + Si + 0.9C + 0.05C2F4 + 0.2NaN3 + 1.7N2 = 4AlN + SiC + 0.2NaF, | (21) |
| 2Al + Si + 0.9C + 0.05C2F4 + 0.2NaN3 + 0.7N2 = 2AlN + SiC + 0.2NaF, | (22) |
| Al + Si + 0.9C + 0.05C2F4 + 0.2NaN3 + 0.2N2 = AlN + SiC + 0.2NaF, | (23) |
| Al + 2Si + 1.8C + 0.1C2F4 + 0.4NaN3 = AlN + 2SiC + 0.4NaF + 0.2N2 , | (24) |
| Al + 4Si + 3.6C + 0.2C2F4 + 0.8NaN3 = AlN + 4SiC + 0.8NaF + 0.7N2 . | (25) |
To achieve an AlN–SiC composition with the maximum silicon carbide phase content (AlN:SiC = 1:4) carbidizing mixtures (B) and (C) with an increased PTFE content were also used:
| Al + 4Si + 3.2C + 0.4C2F4 + 1.6NaN3 = AlN + 4SiC + 1.6NaF + 1.9N2 , | (26) |
| Al + 4Si + 2.8C + 0.6C2F4 + 2.4NaN3 = AlN + 4SiC + 2.4NaF + 3.1N2 . | (27) |
The initial reagent mixtures (charges) from equations (21)–(27), with an average mass of 22 g, were burned in a 4.5 L azide SHS reactor under a nitrogen gas pressure of 3 MPa, both in bulk form in a tracing paper cup with a diameter of 30 mm and height of 45 mm, and as briquettes pressed at a pressure of 7 MPa with a diameter of 30 mm and an average height of 22 mm. Combustion was initiated with an electric tungsten coil. The maximum gas pressure generated in the reactor during combustion was recorded using a manometer, and the maximum combustion temperature was measured with a tungsten-rhenium thermocouple of 200 μm in diameter, inserted into the charge. The cooled combustion product was removed from the reactor, ground to a loose powder state in a porcelain mortar and washed with water to remove the by-product sodium fluoride (NaF).
The phase composition of the synthesized products was determined using an ARL X’TRA powder X-ray diffractometer (Thermo Fisher Scientific, Switzerland) equipped with a copper anode X-ray tube. Diffraction pattern analysis and quantitative phase composition assessment were performed using the Rietveld method in the HighScore Plus software with the COD-2024 crystallographic database. The morphology and particle size of the synthesized compositions were examined using a JSM-6390A scanning electron microscope (Jeol, Japan).
Results and discussion
The results of experimental determination of the parameters for azide SHS of AlN–SiC powder compositions according to equations (21)–(25) are presented in Table 2. As the data indicate, combustion of bulk mixtures occurs more intensively than that of pressed mixtures, with higher maximum temperatures and pressure spikes, accompanied by greater dispersion of the SHS reactants and products. The higher the aluminum content in the charge, the greater the combustion parameters (temperature and pressure); however, at maximum aluminum content, the mass loss of the product due to dispersion is offset by mass gain from increased absorption of gaseous nitrogen during aluminum nitride formation. Mass loss is highest in the synthesis of AlN–SiC compositions with roughly equal molar ratios, especially in the case of bulk mixtures. However, this loss decreases as the SiC content in the combustion product increases, accompanied by a reduction in combustion parameters.
Table 2. Combustion parameters of initial powder mixtures for reactions (21)–(25)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
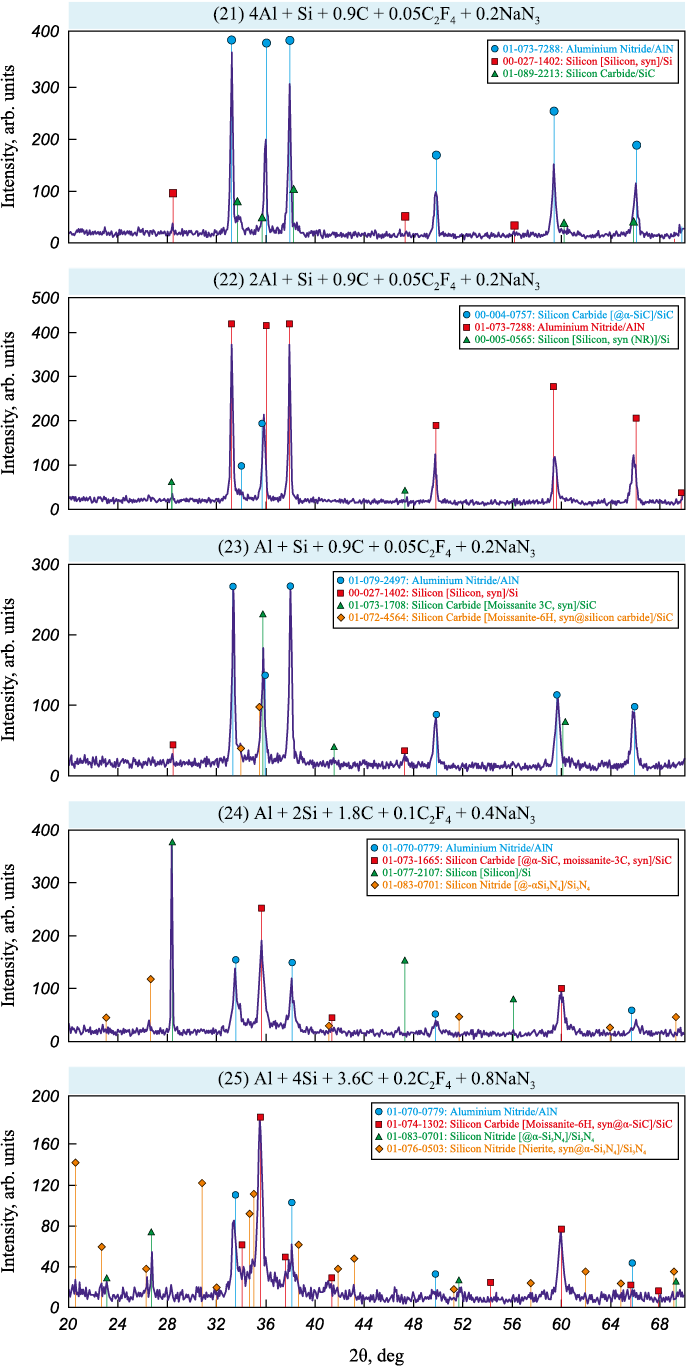
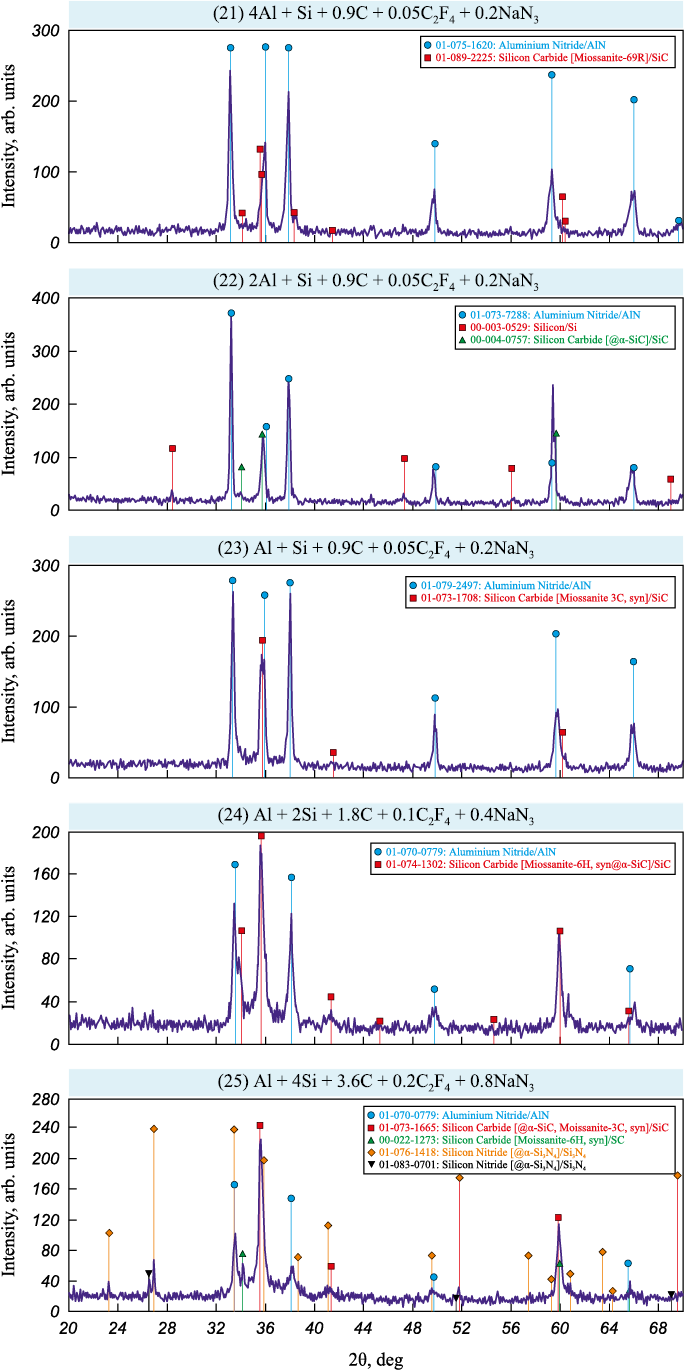
The X-ray diffraction (XRD) patterns of phase analysis of water-washed solid combustion products from bulk and pressed mixtures are shown in Figs. 2 and 3, respectively.
Fig. 2. XRD patterns of combustion products from bulk charges (21)–(25)
Fig. 3. XRD patterns of combustion products from pressed charges (21)–(25) |
The data in Fig. 2 show that the XRD patterns of water-washed combustion products from bulk charges used for synthesizing AlN–SiC composites with higher and equal molar content of the AlN phase, i.e., AlN:SiC = 4:1, 2:1 and 1:1, contain strong reflections only for the target phases AlN and SiC, along with weak reflections from free silicon impurities. For the combustion products with increased SiC phase content, i.e., AlN:SiC = 1:2 and 1:4, in addition to the AlN, SiC, and Si reflections, distinct peaks of the undesirable by-product phase Si3N4 appear, particularly noticeable in the sample with the maximum SiC content. The XRD patterns of combustion products from pressed charges shows only the target phases AlN and SiC for four ratios AlN:SiC = 4:1, 2:1, 1:1 and 1:2 (with a Si impurity for AlN:SiC = 2:1) and the appearance of an additional undesirable by-product phase, Si3N4 , in one case, AlN:SiC = 1:4, with maximum SiC content (Fig. 3).
Table 3 presents the results of quantitative analysis of the XRD patterns, showing the quantitative phase content in the washed combustion products from charges (21)–(25) with carbidizing mixture (A) and minimal PTFE content, as well as for equations (26) and (27) to obtain an AlN–SiC composition with the maximum silicon carbide phase content (AlN:SiC = 1:4) using mixtures (B) and (C) with increased PTFE content. These experimental data are compared with the theoretical results for the target phases AlN and SiC content in the reaction products according to stoichiometric equations (21)–(27).
Table 3 shows that, compared to the azide SHS products without PTFE presented in Table 1, the use of equations (21)–(25) with the first carbidizing mixture (A), containing minimal PTFE (0.9 + 0.05C2F4 ), led to several notable changes:
– the undesirable, water-insoluble impurity of cryolite salt Na3AlF6 completely absent from the combustion products of both bulk and pressed charges, which represents a significant improvement;
– the presence of the undesirable Si3N4 by-product phase is eliminated or substantially reduced to 15 % (observed in bulk charges with AlN:SiC ratios of 1:2 and 1:4, and in pressed charges with an AlN:SiC ratio of 1:4);
– impurities of free silicon and carbon are reduced or even absent, particularly in pressed charges, where only 2 % free silicon was detected at an AlN:SiC ratio of 2:1;
– the experimental content of the target phases AlN and SiC for most ratios closely aligns with the theoretical content, especially in pressed charges, with the exception of bulk charges at AlN:SiC ratios of 1:2 and 1:4 and pressed charges at an AlN:SiC ratio of 1:4.
Table 3. Theoretical and experimental phase composition
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
At the same time, increasing the amount of PTFE in carbidizing mixtures (B) and (C), along with a higher proportion of sodium azide NaN3 for fluorine neutralization in equations (26) and (27), leads to the appearance of the undesirable cryolite salt impurity Na3AlF6 (from 4 to 8 %) in the combustion products of both bulk and pressed charges. It also significantly increases the content of the undesirable Si3N4 by-product phase and, consequently, substantially reduces the proportion of the target phases AlN and SiC compared to their theoretical content.
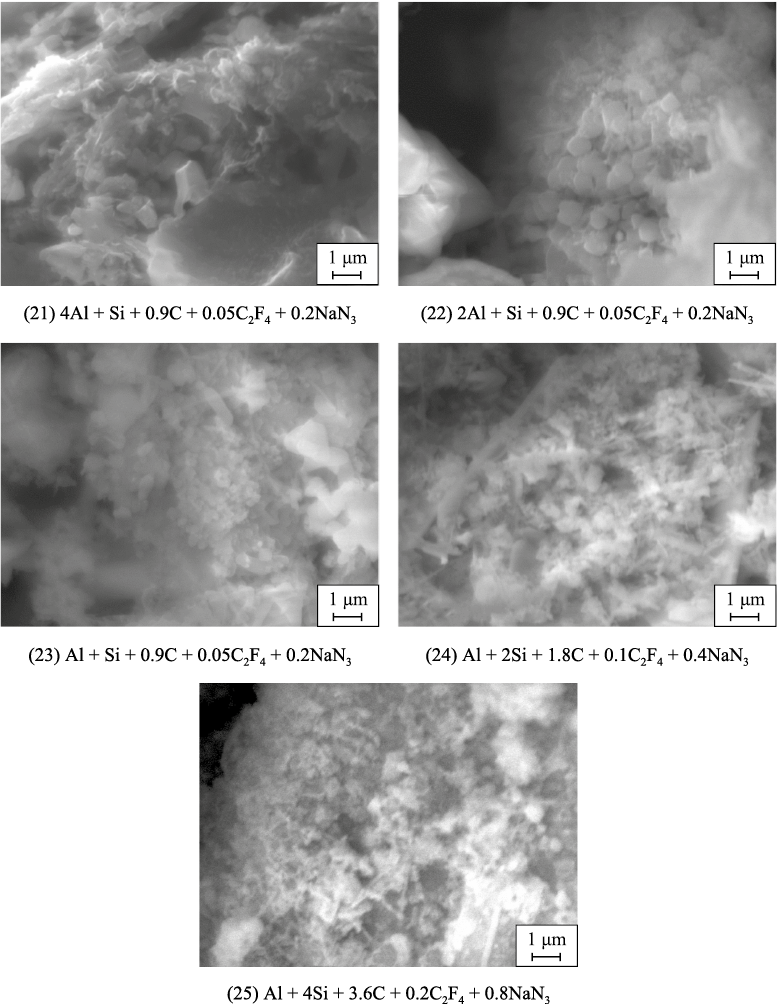
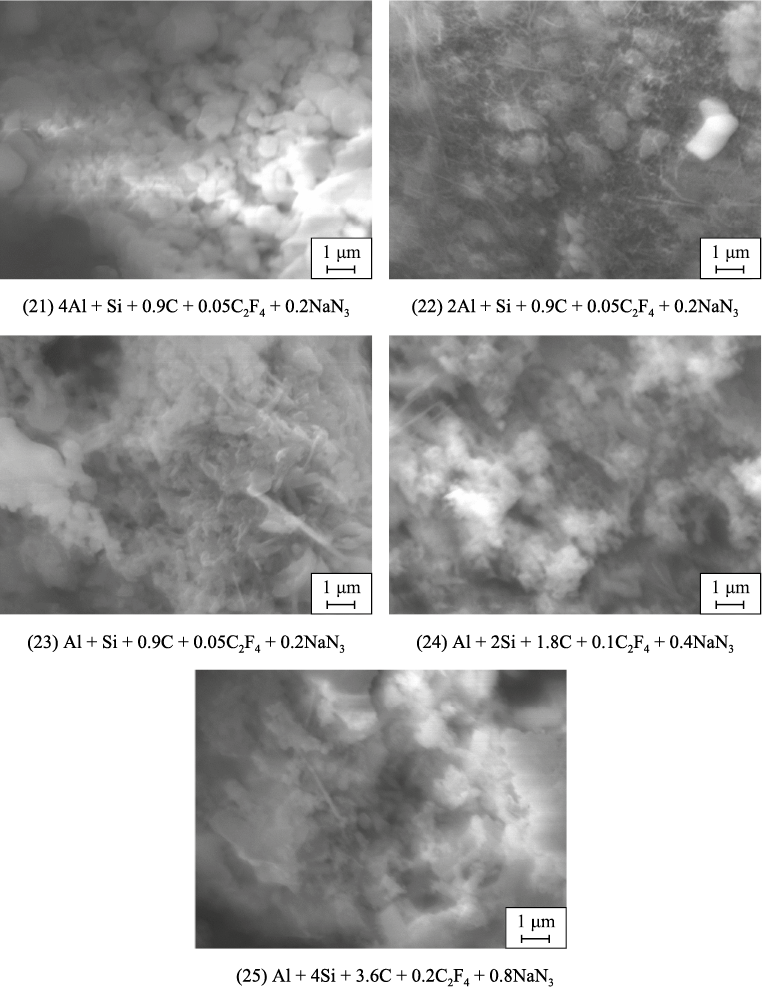
The microstructures of the washed powder combustion products from bulk and pressed charges according to the stoichiometric reaction equations (21)–(25) are shown in Figs. 4 and 5. These figures reveal a trend of decreasing particle size in the combustion products as the SiC content increases in the AlN–SiC powder compositions, corresponding to the trend of decreasing maximum combustion temperature as the SiC content in the product rises (see Table 2). The powders are largest at an AlN:SiC ratio of 4:1, with particles up to 5 μm for bulk charges and up to 2 μm for pressed charges. In other compositions with lower AlN content, the synthesized powders are highly dispersed, consisting of submicron equiaxed particles ranging from 100 nm to 1 μm and nanofibers with diameters of 50–500 nm and lengths up to 5 μm. As the SiC content in the product increases, the proportion of the smallest equiaxed particles, measuring 100–300 nm, also increases. The AlN:SiC ratio of 1:4 composition, with the highest silicon carbide content, forms agglomerates of these small particles.
Fig. 4. SEM images of combustion products from bulk charges (21)–(25)
Fig. 5. SEM images of combustion products from pressed charges (21)–(25) |
Nanofibers are practically absent in compositions with a high AlN content in the combustion products of both bulk and pressed charges, as well as in compositions with a high SiC content in the combustion products of pressed charges. The highest presence of nanofibers is observed in compositions with an equal phase content of AlN and SiC in the combustion products of both types of charges, as well as in compositions with a high SiC content in the combustion products of bulk charges.
Conclusion
The results of this study indicate that incorporating polytetrafluoroethylene (PTFE) in azide SHS technology has a highly positive impact on the production of AlN–SiC ceramic powders. While the traditional approach of azide SHS using sodium azide (NaN3 ) as a nitriding agent and activating halide salt additives (NH4)2SiF6 , AlF3 , and NH4F enabled the production of highly dispersed AlN–SiC powder compositions with particle sizes below 1 μm from a mixture of elemental aluminum, silicon, and carbon powders – a clear advantage of this technology – the phase composition of these compositions had significant drawbacks. Ideally, the phase composition should match the various targeted theoretical ratios of aluminum nitride and silicon carbide phases in accordance with the initial stoichiometric equations. However, the experimentally obtained target phases AlN and SiC were substantially below the theoretical values, particularly for the SiC phase, whose quantity was, on average, half of the theoretical amount. Additionally, significant amounts of undesirable by-product phases, including silicon nitride and the water-insoluble cryolite salt Na3AlF6 , were present.
Introducing PTFE as an activating and carbidizing additive, partially replacing carbon in the carbidizing mixture 0.9C + 0.05C2F4 during azide SHS, resolved most of the traditional approach’s limitations across various AlN and SiC target ratios.
This adjustment maintained the high dispersity of the synthesized AlN–SiC powders while bringing their phase composition – especially in pressed charges – much closer to the theoretical target. The SiC phase content also increased significantly, and the unwanted by-products of silicon nitride and the water-insoluble cryolite salt Na3AlF6 were eliminated. However, for AlN–SiC compositions with the highest relative SiC content, it was not possible to completely avoid the formation of the silicon nitride by-product phase.
References
1. Kosolapova T.Ya., Andreeva T.V., Bartnitskaya T.B., Gnesin G.G., Makarenko G.N., Osipova I.I., Prilutskii E.V. Nonmetallic refractory compounds. Moscow: Metallurgiya, 1985. 224 p. (In Russ.).
2. Yaguchi H., Ozaki K., Somekawa M. Improvement of cutting tool life by AlN deposition on the tool. The Iron and Steel Institute of Japan International. 2004;44(3):598–602. https://doi.org/10.2355/isijinternational.44.598
3. Wang Z., Zou J., Cai S., Zou Y., Ling L., Liang H., Weimin W., Lv X., Fu Z. Aluminum nitride‐based ceramics with excellent thermal shock resistances. Journal of the American Ceramic Society. 2024;107(8):5352–5363. https://doi.org/10.1111/jace.19829
4. Nepochatov Yu., Zemnitskaya A., Mul’ P. Development of ceramics based on aluminum nitride for electronic products. Sovremennaya elektronika. 2011;(9):14–16. (In Russ.).
5. Li N., Ho C.P., Zhu S., Fu Y.H., Zhu Y., Lee L.Y.T. Aluminium nitride integrated photonics: A review. Nanophotonics. 2021;10(9):2347–2387. https://doi.org/10.1515/nanoph-2021-0130
6. Unni C.K., Gordon D.E. Mechanical properties of monolithic AlN and SiCw/AlN composites. Journal of Materials Science. 1995;30(5):1173–1179. https://doi.org/10.1007/BF00356116
7. Besisa D.H.A., Ewais E.M.M., Ahmed Ya.M.Z., Elhosiny F.I., Fend T., Kuznetsov D.V. Investigation of microstructure and mechanical strength of SiC/AlN composites processed under different sintering atmospheres. Journal of Alloys and Compounds. 2018;756:175–181. https://doi.org/10.1016/J.JALLCOM.2018.05.020
8. Besisa D.H.A., Ewais E.M.M., Ahmed Ya.M.Z., Elhosiny F.I., Fend T., Kuznetsov D.V. Thermal shock resistance of pressureless sintered SiC/AlN ceramic composites. Materials Research Express. 2018;5(1):015506. https://doi.org/10.1088/2053-1591/aaa2c2
9. Zangvil A., Ruh R. Phase relationship in the silicon carbide-aluminum nitride system. Journal of the American Ceramic Society. 1988;71(10):884–890. https://doi.org/10.1111/J.1151-2916.1988.TB07541.X
10. Han J., Li Y., Chenhong M., Zheng Q., Zhang X. Formation mechanism of AlN-SiC solid solution with multiple morphologies in Al-Si-SiC composites under flowing nitrogen at 1300 °C. Journal of the European Ceramic Society. 2022;42(14):6356–6363. https://doi.org/10.1016/j.jeurceramsoc.2022.07.011
11. Miura M., Yogo T., Hirano S.-I. Phase separation and toughening of SiC–AlN solid-solution ceramics. Journal of Materials Science. 1993;28(14):3859–3865. https://doi.org/10.1007/BF00353191
12. Lee R.-R., Wei W.-C. Chapter 39 – Fabrication, microstructure, and properties of SiC–AlN ceramic alloys. In: A Collection of Papers Presented at the 14th Annual Conference on Composites and Advanced Ceramic Materials: Ceramic Engineering and Science Proceedings. 1990. P. 1094–1121. https://doi.org/10.1002/9780470313008.ch39
13. Palmero P. Structural ceramic nanocomposites: A review of properties and powders’ synthesis methods. Nanomaterials. 2015;5(2):656–696. https://doi.org/10.3390/nano5020656
14. Li G., Li B., Ren B., Chen H., Zhu B., Chen J. Synthesis of aluminum nitride using sodium aluminate as aluminum source. Processes. 2023;11(4):1034. https://doi.org/10.3390/pr11041034
15. Gao P., Jia Ch.-Ch., Cao W.-B., Wang C.-C., Liang D., Xu G.-L. Dielectric properties of spark plasma sintered AlN/SiC composite ceramics. International Journal of Minerals, Metallurgy, and Materials. 2014;21(6):589–594. https://doi.org/10.1007/s12613-014-0946-1
16. Elagin A.A., Beketov A.R., Baranov M.V., Shishkin R.A. Aluminum nitride. Preparation methods (Review). Refractories and Industrial Ceramics. 2013;53(6):395–403.
17. Teusel I., Rüssel C. Pressureless sintering of aluminium nitride/silicon carbide ceramics. Journal of Materials Science Letters. 1992;11(4):205–207. https://doi.org/10.1007/bf00741422
18. Ruh R., Zangvil A. Composition and properties of hot-pressed SiC–AlN solid solution. Journal of the American Ceramic Society. 1982;65(5):260–265. https://doi.org/10.1111/J.1151-2916.1982.tb10429.x
19. Du X.L., Qin M.L., Sun Y., Yuan Z.H., Yang B.H., Qu X.H. Structure and thermal conductivity of powder injection molded AlN ceramic. Advanced Powder Technology. 2010;21(4):431–434. https://doi.org/10.1016/j.apt.2010.01.001
20. Kobayashi R., Oh-Ishi K., Tu R., Goto T. Sintering behavior, microstructure, and thermal conductivity of dense AlN ceramics processed by spark plasma sintering with Y2O3-CaO-B additives. Ceramics International. 2015;41(1B):1897–1901. https://doi.org/10.1016/j.ceramint.2014.09.040
21. Xu G.F., Olorunyolemi T., Wilson O.C., Lloyd I.K., Carmel Y. Microwave sintering of high-density, high thermal conductivity AIN. Journal of Materials Research. 2002;17(11):2837–2845. https://doi.org/10.1557/JMR.2002.0412
22. Tang Y., Xue Z., Zhou G., Hu S. Fabrication of high thermal conductivity aluminum nitride ceramics via digital light processing 3D printing. Materials. 2024,17(9):2010. https://doi.org/10.3390/ma17092010
23. Basu B., Balani K. Advanced structural ceramics. Hoboken. New Jersey: John Wiley & Sons, Inc., 2011. 502 p.
24. Camargo P.H.C., Satyanarayana K.G., Wypych F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Materials Research. 2009; 12(1):1–39. https://doi.org/10.1590/S1516-14392009000100002
25. Rane V., Kanny K., Abitha V.K., Thomas S. Chapter 5 – Methods for synthesis of nanoparticles and fabrication of nanocomposites. In: Synthesis of inorganic nanomaterials: Advances and key technologies. A volume in micro and nano technologies. (Eds. S.M. Bhagyaraj, O.S. Oluwafemi, N. Kalarikkal, S. Thomas). Woodhead Publishing, 2018. P. 121–139.
26. https://doi.org/10.1016/B978-0-08-101975-7.00005-1
27. Wu X., Deng C., Di J., Ding J., Zhu H., Yu C. Fabrication of novel AlN–SiC–C refractories by nitrogen gas-pressure sintering of Al4SiC4. Journal of the European Ceramic Society. 2022;42(8):3634–3643. https://doi.org/10.1016/j.jeurceramsoc.2022.02.058
28. Chen K., Jin H., Zhou H., Ferreira J. Combustion synthesis of AlN–SiC solid solution particles. Journal of The European Ceramic Society. 2000;20(14-15):2601–2606. https://doi.org/10.1016/S0955-2219(00)00119-9
29. Rogachev A.S., Mukasyan A.S. Combustion for material synthesis. New York: CRC Press, 2014. 424 p. https://doi.org/10.1201/b17842
30. Levashov E.A., Mukasyan A.S., Rogachev A.S., Shtansky D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. International Materials Reviews. 2016;62(4):1–37. https://doi.org/10.1080/09506608.2016.1243291
31. Xue H., Munir Z.A. The synthesis of composites and solid solutions of α-SiC AlN by field-activated combustion. Scripta Materialia. 1996;35(8):979–982. https://doi.org/10.1016/1359-6462(96)00246-1
32. Abbasi Z., Shariat M.H., Javadpour S. Microwave-assisted combustion synthesis of AlN–SiC composites using a solid source of nitrogen. Powder Technology. 2013;249:181–185. https://doi.org/10.1016/j.powtec.2013.08.012
33. Borovinskaya I.P., Akopdzhanyan T.G., Chemagina E.A., Sachkova N.V. Solid solution (AlN)x(SiC)1-x (x = 0.7) by SHS under high pressure of nitrogen gas. International Journal of Self-Propagating High-Temperature Synthesis. 2018;27(1):33–36. https://doi.org/10.3103/S1061386218010028
34. Juang R.-C., Chen C.-C., Kuo J.-C., Huang T.-Y., Li Y.-Y. Combustion synthesis of hexagonal AlN–SiC solid solution under low nitrogen pressure. Journal of Alloys and Compounds. 2009;480(2):928–933. https://doi.org/10.1016/j.jallcom.2009.02.102
35. Amosov A.P., Titova Yu.V., Belova G.S., Maidan D.A., Minekhanova A.F. SHS of highly dispersed powder compositions of nitrides with silicon carbide. Review. Powder Metallurgy and Functional Coatings. 2022;16(4):34–57. https://doi.org/10.17073/1997-308X-2022-4-34-57
36. Titova Yu.V., Amosov A.P., Maidan D.A., Belova G.S., Minekhanova A.F. Physical and chemical features of combustion synthesis of nanopowder composition AlN–SiC using sodium azide. AIP Conference Proceedings. 2020;2304(1):020008. https://doi.org/10.1063/5.0034318
37. Amosov A., Smetanin K., Titova Yu., Maidan D. Preparation of ceramic nitride-carbide composition AlN–SiC by SHS method using halide salt and sodium azide. In: Proceedings of 7th International Congress on Energy Fluxes and Radiation Effects (EFRE-2020) (Tomsk, Russia, September 14–25, 2020). IEEE Xplore. 2020:1110–1114. https://doi.org/10.1109/EFRE47760.2020.9241986
38. Belova G.S. Self-propagating high-temperature synthesis of ceramic nitride-carbide highly dispersed powder compositions Si3N4–SiC, AlN–SiC and TiN–SiC using sodium azide and halide salts: Diss. Cand. Sci. (Eng.). Samara: SamSTU, 2022. 209 p. (In Russ.).
39. Mukasyan A.S. Combustion synthesis of silicon carbide. In: Properties and applications of silicon carbide. (Ed. R. Gerhardt). Rijeka, Croatia: InTech, 2011. P. 361–388.
40. Nersisyan G.A., Nikogosov V.N., Kharatyan S.L., Merzhanov A.G. Chemical transformation mechanism and combustion regimes in the system silicon-carbon-fluoroplastic. Combustion, Explosion, and Shock Waves. 1991;27(6):720–724. https://doi.org/10.1007/BF00814517
41. Kharatyan S.L., Nersisyan H.H. Chemically activated SHS in synthesis of refractory carbide powders. Key Engineering Materials. 2002;217:83–92. https://doi.org/10.4028/www.scientific.net/KEM.217.83
42. Khachatryan G.L., Arutyunyan A.B., Kharatyan S.L. Activated combustion of a silicon–carbon mixture in nitrogen and SHS of Si3N4–SiC composite ceramic powders and silicon carbide. Combustion, Explosion, and Shock Waves. 2006;42(5):543–548. https://doi.org/10.1007/S10573-006-0086-7
43. Amirkhanyan N., Kirakosyan H., Zakaryan M., Zurnachyan A., Rodriguez M.A., Abovyan L., Aydinyan S. Sintering of silicon carbide obtained by combustion synthesis. Ceramics International. 2023;49(15):26129–26134. https://doi.org/10.1016/j.ceramint.2023.04.233
44. Zakorzhevsky V.V., Loryan V.E., Akopdzhanyan T.G. Self-propagating high-temperature synthesis of silicon carbide nanofibers. Russian Journal of Non-Ferrous Metals. 2020;61(6):675–679. https://doi.org/10.3103/S106782122006022X
45. Vorotilo S., Levashov E.A., Potanin A.Yu., Loginov P.A., Shvyndina N.V. Features of synthesizing ceramic composites discretely reinforced by carbon fibers and SiC nanowires formed in situ in the combustion wave. Russian Journal of Non-Ferrous Metals. 2020;61(5):559–570. https://doi.org/10.3103/S1067821220050168
About the Authors
A. P. AmosovRussian Federation
Aleksandr P. Amosov – Dr. Sci. (Phys.-Math.), Professor, Head of the Department of Metallurgy, Powder Metallurgy, Nanomaterials
244 Molodogvardeyskaya Str., Samara 443100, Russia
Yu. V. Titova
Russian Federation
Yuliya V. Titova – Cand. Sci. (Eng.), Associate Professor of the Department of the Department of Metallurgy, Powder Metallurgy, Nanomaterials
244 Molodogvardeyskaya Str., Samara 443100, Russia
I. A. Uvarova
Russian Federation
Irina A. Uvarova – Junior Researcher of the Research Sector of the Department of the Department of Metallurgy, Powder Metallurgy, Nanomaterials
244 Molodogvardeyskaya Str., Samara 443100, Russia
G. S. Belova
Russian Federation
Galina S. Belova – Cand. Sci. (Eng.), Associate Professor of the Department of the Department of Metallurgy, Powder Metallurgy, Nanomaterials
244 Molodogvardeyskaya Str., Samara 443100, Russia
Review
For citations:
Amosov A.P., Titova Yu.V., Uvarova I.A., Belova G.S. Azide self-propagating high-temperature synthesis of a highly dispersed AlN–SiC powder composition using polytetrafluoroethylene. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):28-43. https://doi.org/10.17073/1997-308X-2024-6-28-43