Scroll to:
Obtaining ceramics from boron carbide with chromium-based additives (Cr3C2, CrB2)
https://doi.org/10.17073/1997-308X-2025-3-5-14
Abstract
This study presents the results of spark plasma sintering of powders within the boron–carbon–chromium system, focusing on boron carbide (B4C), chromium carbide (Cr3C2 ), and chromium diboride (CrB2 ). The powders were synthesized using the original vacuum-free direct current arc reactor, where the starting powder mixture was exposed to an arc discharge for 60 s under a direct current of 200 A. Bulk samples based on B4C and CrB2 were sintered under identical conditions, with a temperature of 1800 °C and a pressure of 60 MPa, while the sintering of Cr3C2-based ceramics was conducted at 1300 °C and 30 MPa. In some cases, sintering additives – 25 wt. % Cr3C2 and 20 wt. % CrB2 – were introduced during the sintering of B4C-based bulk samples. The phase composition of the sintered samples was analyzed using X-ray diffraction (XRD), while the microstructure and elemental composition were examined via scanning electron microscopy (SEM). The hardness of the sintered ceramics was measured using a Vickers indenter under a load of 1 kg, revealing hardness values of 22.7 ± 1.8 GPa for B4C, 12.6 ± 0.3 GPa for CrB2 , and 11.4 ± 0.1 GPa for Cr3C2 . The introduction of 25 wt. % Cr3C2 as a sintering additive in B4C-based ceramics reduced the hardness to 17.7 ± 5.6 GPa; however, it significantly improved the fracture toughness, increasing it from 2.5 ± 0.2 to 3.3 ± 0.3 MPa·m1/2. Conversely, the addition of 20 wt. % CrB2 during B4C sintering led to an increase in the bulk sample’s hardness from 22.7 ± 1.8 GPa to 26.8 ± 1.3 GPa.
Keywords
For citations:
Vassilyeva Yu.Z., Povalyaev P.V., Kuznetsova A.A., Pak A.Ya. Obtaining ceramics from boron carbide with chromium-based additives (Cr3C2, CrB2). Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(3):5-14. https://doi.org/10.17073/1997-308X-2025-3-5-14
Introduction
Boron carbide (B4C) is one of the most promising superhard materials in its class, widely recognized for its unique properties, including a high melting point (~2450 °C according to the boron carbide phase diagram) and excellent thermal stability [1]. B4C-based ceramics exhibit outstanding hardness (~29 GPa) combined with a relatively low density (2.52 g/cm3) [2]. These properties make boron carbide highly suitable for a range of applications, such as the production of refractory materials, abrasive products, neutron radiation absorbers [1], coatings for cutting tools [3], and ballistic protection systems [4].
The performance of B4C-based ceramics can be enhanced through the introduction of sintering additives. Numerous studies have explored how various additives influence the mechanical properties of boron carbide ceramics. Research findings [5–8] indicate that the addition of carbides and borides of silicon and chromium can significantly improve these properties. For example, study [6] demonstrated that adding Cr3C2 as a sintering additive to B4C significantly increases the densification while achieving a high relative density (up to 95 %) and flexural strength (up to 440 MPa). Previously published studies [5; 9] have shown that the introduction of chromium diboride as a sintering additive can enhance the mechanical properties of boron carbide ceramics. In [5], the addition of 20 mol. % CrB2 as a sintering additive increased the fracture toughness of the sintered ceramic to 3.5 MPa·m1/2, with a flexural strength of 630 MPa. Similarly, study [9] reported that a B4C–10mol.%CrB2 composite exhibited a fracture toughness of 5.25 MPa·m1/2 (compared to 4.33 MPa·m1/2 without additives) and a microhardness of 37.1 GPa (compared to 35.5 GPa without additives).
The primary methods for producing boron carbide include carbothermal reduction of boron oxide [2; 10], self-propagating high-temperature synthesis [11; 12], mechanical activation followed by heat treatment [13], among others. Among advanced synthesis methods, the arc discharge technique stands out due to its ability to achieve extremely high temperatures over a wide range, with rapid heating rates [14]. In the authors’ previous studies, the feasibility of synthesizing boron carbide using a vacuum-free arc discharge method with a horizontal discharge circuit was demonstrated. This approach eliminates the need for creating an inert atmosphere in the reaction zone or ensuring airtight conditions in the reaction chamber, as the vacuum-free arc reactor can operate in ambient air. This significantly simplifies the reactor design and reduces the overall processing cycle time [15].
The objective of this study is to investigate the synthesis of boron carbide, chromium diboride, and chromium carbide powders using the vacuum-free arc discharge method, followed by the sintering of the obtained powders. A key focus of this work is the evaluation of the mechanical properties of the resulting ceramic samples and the assessment of how sintering additives, specifically CrB2 and Cr3C2 compounds, influence the mechanical performance of B4C-based ceramic composites.
Research materials and methods
The synthesis of powders (B4C, Cr3C2 , CrB2 ) for sintering was performed using a vacuum-free arc discharge method in a custom-designed reactor with a modified discharge circuit configuration. The schematic diagram of the reactor, along with a detailed description of the synthesis process and material characteristics, can be found in studies [16–18]. The reactor is equipped with a direct current (DC) power source capable of regulating the current within a range of 20 to 220 A. The negative terminal of the power source was connected to an aluminum plate with openings designed to hold graphite crucibles, while the positive terminal was connected to a vertically positioned steel sleeve mounted above the aluminum plate. Starting powders were placed inside the cavity of the graphite crucible and sealed with a graphite lid. The arc discharge was initiated by establishing contact between the electrode and the crucible lid, after which the discharge was maintained for a predetermined duration before being terminated. The operating parameters of the arc reactor used during the synthesis of the powder materials are summarized in Table 1.
Table 1. Parameters of synthesis of powder materials
|
The ceramic samples were sintered using the spark plasma sintering (SPS) method with a GT Advanced Technologies SPS 10-4 system. During the fabrication process, the powder materials were simultaneously pressed and sintered under vacuum conditions. Five bulk cylindrical samples, each with a diameter of 12.7 mm and a height of 3 mm, were sintered under conditions selected based on literature data, as detailed in Table 2. The sintered samples included tablets made from boron carbide powders, as well as boron carbide with the addition of chromium carbide and chromium diboride. Before sintering each bulk sample, the starting powders or powder mixtures for each composition were milled in a tungsten carbide ball mill for 5 min.
The phase composition of the synthesized and sintered products was analyzed using X-ray diffraction (XRD) on a Shimadzu XRD 7000 diffractometer with CuKα1 radiation (λ = 1.54060 Å). The microstructure and morphology of the synthesized and sintered materials were examined using a Tescan Vega 3 SBU scanning electron microscope (SEM) equipped with an Oxford X-Max 50 energy-dispersive X-ray spectroscopy (EDS) system featuring an Si/Li crystal detector. Transmission electron microscopy (TEM) was performed using a JEOL JEM 2100F microscope equipped with an EX-24063JGT EDS detector to analyze finer structural details.
The density of the sintered ceramic samples was measured using the hydrostatic weighing method with a specialized attachment for HR-250AZ analytical balances (A&D Company) in distilled water. The hardness of the sintered ceramics was evaluated using a Pruftechnik KB-30S microhardness tester with a Vickers indenter under a load of 1 kg. The fracture toughness of the bulk samples was determined using the indentation method, by measuring the lengths of cracks emanating from the corners of the Vickers indentation marks, as observed in SEM images. These cracks were generated under the same 1 kg load applied with the Pruftechnik KB-30S microhardness tester.
Research results and discussion
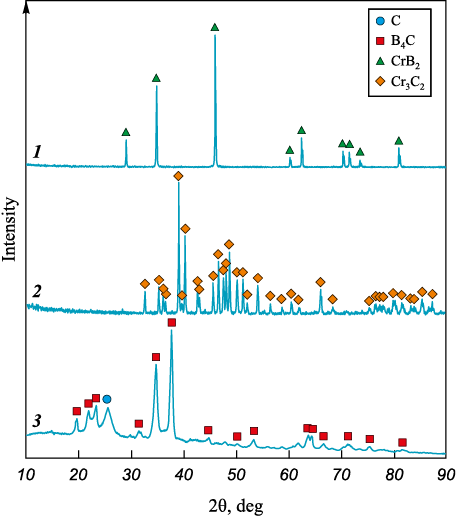
The X-ray diffraction analysis (Fig. 1) of the synthesized powder confirms the formation of boron carbide (B4C) (JCPDS No. 35-798, space group R-3m, rhombohedral crystal system) as a result of exposure to the thermal field generated by an arc discharge initiated in air under normal atmospheric pressure, with a current of 200 A and an exposure time of 60 s applied to the boron–carbon mixture. Notably, traces of unreacted elemental boron were detected in the XRD pattern, indicated by a broad halo within the angular range of 2θ = 10÷20°. In addition, a diffraction peak at 2θ ≈ 26.1°, corresponding to the graphite phase, was identified in the sample.
Fig. 1. Typical X-ray diffraction patterns of the synthesized CrB2 , Cr3C2 and B4C powders |
Fig. 1 also shows the XRD patterns of the synthesized chromium carbide and chromium diboride powders. According to the diffraction data, the chromium carbide powder consists of a crystalline Cr3C2 phase (JCPDS No. 35-0804, orthorhombic structure), while the chromium diboride powder corresponds to the CrB2 phase (JCPDS No. 34-369, hexagonal structure).
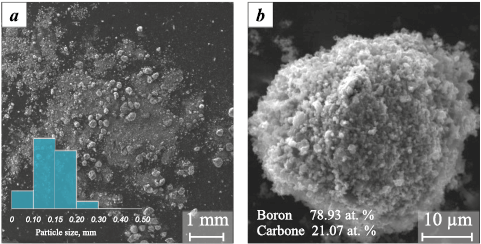
The morphology of the synthesized boron carbide powder was examined using scanning electron microscopy (SEM). A representative SEM image is shown in Fig. 2.
Fig. 2. Typical scanning electron microscopy images of the boron carbide sample |
The newly formed boron carbide particles appear as agglomerates with a broad size distribution ranging from ~100 to ~500 µm, with the most frequent particle size falling within the 100–150 µm range (Fig. 2, a, inset). According to elemental analysis, typical particles contain boron (78.93 at. %) and carbon (21.07 at. %), with an atomic ratio consistent with the stoichiometry of boron carbide (B4C), as previously confirmed by XRD analysis. A small amount of impurities (not exceeding 2.0 at. %) was also detected in the samples.
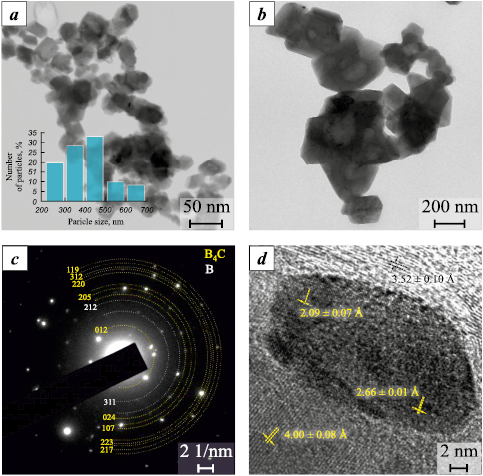
Fig. 3 shows the results of transmission electron microscopy of the nanoscale fraction of the synthesized boron carbide (B4C) powder. As can be seen, the newly formed particles exhibit faceted features and a size distribution ranging from 200 to 700 nm, with most particles falling within the 400–500 nm range. The electron diffraction pattern (Fig. 3, c) reveals interplanar spacings of 3.80, 1.89, 1.71, 1.62, 1.40, 1.32, 1.31, 1.26, and 1.21 Å, which, within the permissible measurement error, correspond to the reference interplanar spacings of the B4C phase (JCPDS No. 35-798, PDF-4+). Additionally, interplanar spacings of 2.34 and 2.09 Å were identified, corresponding within the measurement error to the reference values for the B13C2 phase (JCPDS No. 71-108, PDF-4+). The identified B13C2 phase represents a structural variant of boron carbide (B4C) and is a hyperstoichiometric phase relative to B4C. Its formation is likely due to the development of localized regions with non-uniform distribution of the starting powders in the reaction zone [19].
Fig. 3. Typical transmission electron microscopy results |
Ceramic samples based on boron carbide (B4C), chromium carbide (Cr3C2 ), and chromium diboride (CrB2 ) were subsequently fabricated using the spark plasma sintering (SPS) method. Based on previous studies [5–8], it has been established that the introduction of sintering additives in the form of chromium carbide and chromium diboride enhances the mechanical properties of the final ceramic products. According to the results of study [5], adding 20 wt. % CrB2 during the sintering of B4C-based ceramic samples leads to composites with the highest fracture toughness and hardness. In contrast, the addition of 25 wt. % Cr3C2 results in samples with the highest density [6], which also contributes to improved mechanical properties of the bulk material. Therefore, to enhance the mechanical properties of the ceramics, samples were prepared from powders with the following additives: B4C + 20 wt. % CrB2 and B4C + 25 wt. % Cr3C2 . To evaluate the effect of sintering additives on the properties of bulk ceramics, the characteristics of samples made from pure powders were compared with those of sintered samples containing additives. Chromium carbide and chromium diboride powders synthesized via the vacuum-free arc discharge method were used as sintering additives.
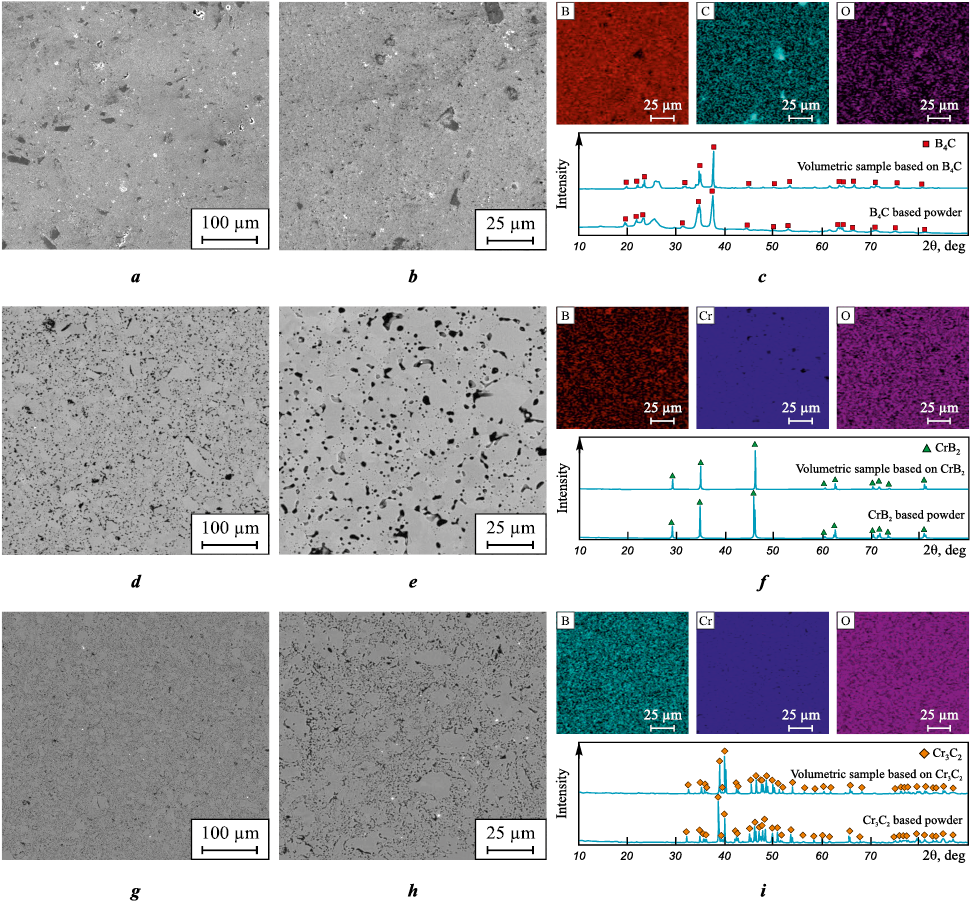
Fig. 4 presents scanning electron microscopy images of the sintered ceramic samples based on B4C, CrB2 , and Cr3C2 . According to the XRD patterns, no phase transitions were detected after sintering, and the phase composition remained nearly identical to that of the powders synthesized by the vacuum-free arc discharge method.
Fig. 4. Typical SEM images in backscattered electron mode with elemental distribution maps |
The surface of the bulk boron carbide (B4C) sample (Fig. 4, a–c) exhibits distinct structural features, particularly regions with multiple clustered carbon particle (agglomerates) reaching up to up to ~17 µm in size. Additionally, changes in the phase composition of the bulk sample were observed compared to the starting powder. According to the X-ray diffraction analysis (Fig. 4, c), the diffraction halo within the angular range of 2θ = 10÷20°, corresponding to amorphous boron particles, becomes less pronounced after sintering. This change in phase composition can be attributed to the reaction between amorphous boron and carbon particles during the sintering process, leading to the formation of boron carbide (B4C) and the agglomeration of free carbon (Fig. 4, c, carbon distribution map).
Elemental analysis (Fig. 4, c) shows that the boron carbide sample contains boron (64.90 at. %) and carbon (29.03 at. %), with an atomic ratio consistent with the B4C stoichiometry previously determined by XRD. In addition, trace amounts of oxide impurities (no more than 2.0 at. %) were identified in the sample. Elemental distribution maps also reveal the presence of oxide compounds on the sample surface (Fig. 4, c), which likely originate from the starting powders, such as boron oxide (B2O3 ) particles present in the raw boron powder. However, the identification of these compounds via XRD is challenging due to their low concentration relative to the total sample volume.
SEM images of the chromium diboride (CrB2 ) ceramic (Fig. 4, d–f ) reveal light and dark globular regions on the sample surface at low magnifications, associated with the distribution of the primary components. Boron particle clusters up to ~10 µm in size were also identified on the CrB2 sample surface. Elemental analysis (Fig. 4, f) indicates that the sample contains boron (69.80 at. %) and chromium (28.46 at. %) in an atomic ratio corresponding to the stoichiometry of CrB2 . A small amount of oxide compounds (not exceeding 2.0 at. %) was also detected on the sample surface.
The surface of the pure chromium carbide (Cr3C2) ceramic sample (Fig. 4, g–i) also shows dark regions in the SEM images, corresponding to carbon particle agglomerates with sizes up to 5 µm. Elemental analysis confirms a uniform distribution of chromium and carbon, with average contents of 48.54 at. % (C) and 48.52 at. % (Cr). A small amount of oxide compounds (not exceeding 3.0 at. %) was also identified.
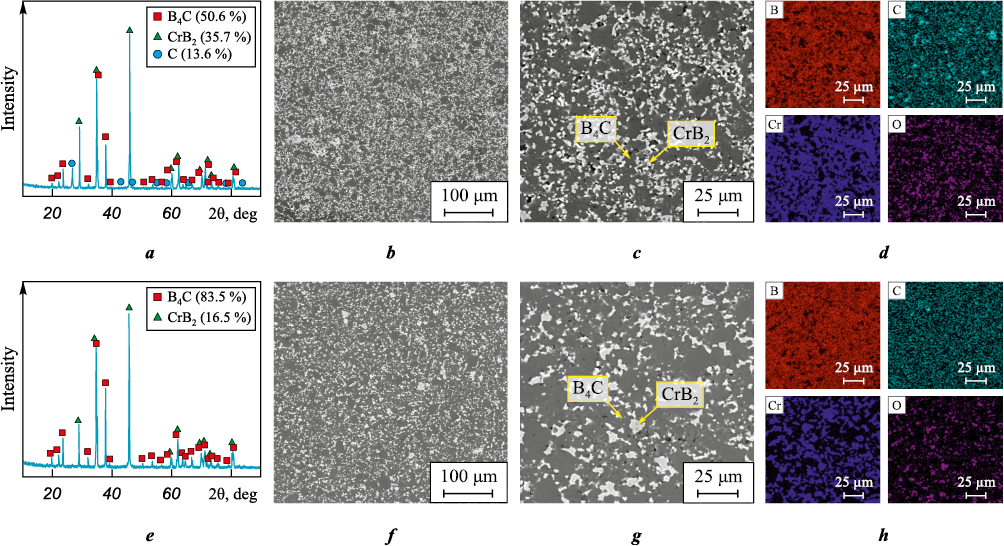
Fig. 5 presents SEM images and XRD results of the sintered boron carbide-based ceramic samples with additives (20 wt. % CrB2 and 25 wt. % Cr3C2 ).
Fig. 5. Typical X-ray diffraction patterns and backscattered electron SEM images |
According to the X-ray diffraction analysis (Fig. 5, a), the sample sintered from boron carbide powder with the addition of 25 wt. % chromium carbide contains CrB2 (JCPDS No. 89-3533), B4C (JCPDS No. 35-798), and C (JCPDS No. 75-3078) phases. The formation of chromium diboride during the sintering of the B4C + 25 wt. % Cr3C2 composite is attributed to the diffusion of boron atoms from the boron carbide phase into the chromium carbide phase at high temperatures. This process results in the formation of CrB2 and the release of free carbon from B4C and Cr3C2 compounds. The free carbon can either react with boron atoms to form additional boron carbide phases or accumulate in localized carbon-rich regions, as indicated by the carbon distribution map (Fig. 5, c). The formation of chromium diboride (CrB2 ) in this system follows the chemical reaction [20]:
3B4C + 2Cr3C2 → 6CrB2 + 7C.
The sample surface (Fig. 5, b, c) displays both light and dark regions. The light regions correspond to the CrB2 phase, while the darker areas are associated with boron carbide. Additionally, pores up to 10 µm in size were observed.
Elemental analysis (Fig. 5, d) shows that the light regions represent the chromium diboride phase with traces of carbon. These areas contain carbon (20.57 at. %), boron (68.47 at. %), and chromium (10.96 at. %), confirming the presence of CrB2 . A small amount of oxide compounds (up to 1.10 at. %) was also detected. According to the elemental analysis results, the dark regions correspond to the boron carbide phase, with elemental analysis revealing boron (72.11 at. %), carbon (24.5 at. %), and chromium (2.96 at. %), which is consistent with the B4C phase composition. Minor impurities (up to 1.0 at. %) were also present in the sample.
Fig. 5, e–h shows the XRD results and SEM images of the sintered ceramic sample made from boron carbide powder with the addition of 20 wt. % chromium diboride, captured in secondary electron mode. The diffraction peaks observed in the XRD pattern (Fig. 5, e) correspond to the characteristic peaks of the crystalline phases CrB2 (JCPDS No. 89-3533) and B4C (JCPDS No. 35-798).
The surface of the B4C + 20 wt. % CrB2 sample (Fig. 5, f, g) features both light and dark regions, along with pores up to 5 µm in size. The sample also displays distinct structural features, including carbon particle clusters measuring up to 4 µm.
Elemental analysis (Fig. 5, h) reveals that the light regions contain boron (68.3 at. %), chromium (17.21 at. %), and carbon (12.99 at. %), indicating the presence of chromium diboride grains (Fig. 5, e). Oxygen was also detected in these regions, with a concentration of up to 1.5 at. %. In contrast, the dark regions contain boron (75.66 at. %), carbon (22.30 at. %), and chromium (1.66 at. %), suggesting these areas correspond to boron carbide grains (Fig. 5, g). Trace amounts of oxide compounds (up to 1.0 at. %) were also identified in these regions.
The ceramic samples were subjected to Vickers hardness testing under a load of 1 kg. The test results, along with the sample characteristics, are presented in Table 2.
Table 2. Sintering parameters and characteristics of ceramic samples
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The theoretical density of the composite materials was calculated based on the phase composition of the bulk ceramic samples. Quantitative analysis of the crystalline phases (Fig. 5, a, e) was performed using PowderCell 2.4 software. Following this analysis, the theoretical density of the composite materials was determined using the density values of the identified phases (B4C, CrB2 , and C).
The data presented in Table 2 reveals the following hardness trend: B4C + 20 wt. % CrB2 > В4С > B4C + 25 wt. % Cr3C2 > CrB2 > Cr3C2 , specifically 26.8 > 22.7 > 17.7 > 12.6 > 11.4 GPa. The introduction of chromium diboride as a sintering additive led to an increase in the relative density of the bulk ceramic sample from 95.2 to 96.2 %, which is consistent with experimental data from [25]. This increase in density also contributed to improved mechanical properties, as reflected by the rise in hardness from 22.7 to 26.8 GPa.
The addition of chromium carbide during the sintering process resulted in a decrease in both the density and mechanical properties of the ceramic, reducing the hardness to 17.7 GPa. This decrease in relative density is attributed to the formation of porous regions containing a significant amount of free carbon. For the B4C sample with Cr3C2 addition, fracture toughness was also investigated. The results showed that the fracture toughness of pure boron carbide was 2.5 ± 0.2 MPa·m1/2, while the introduction of 25 wt. % Cr3C2 increased this value to 3.3 ± 0.3 MPa·m1/2, which is comparable to the results reported in [5; 9].
Conclusion
In this study, the fabrication of bulk ceramics based on compounds from the boron–carbon–chromium system was successfully demonstrated. The powders used for sintering the bulk samples included boron carbide (B4C), chromium carbide (Cr3C2 ), and chromium diboride (CrB2 ). Notably, these powders were synthesized using a vacuum-free direct current arc reactor specifically developed by the authors of this research. The hardness of the B4C-based ceramic was measured at 22.7 GPa, and the incorporation of 20 wt. % CrB2 as a sintering additive led to an increase in hardness to 26.8 GPa. Furthermore, the addition of 25 wt. % Cr3C2 enhanced the fracture toughness from 2.5 to 3.3 MPa·m1/2. The hardness of ceramics based on chromium carbide and chromium diboride was found to be 11.4 and 12.6 GPa, respectively.
References
1. Domnich V., Reynaud S., Haber R.A., Chhowalla M. Boron carbide: Structure, properties, and stability under stress. Journal of the American Ceramic Society. 2011; 94(11):3605–3628. https://doi.org/10.1111/j.1551-2916.2011.04865.x
2. Roy T.K., Subramanian C., Suri A.K. Pressureless sintering of boron carbide. Ceramics International. 2006; 32(3):227–233. https://doi.org/10.1016/j.ceramint.2005.02.008
3. Datye A., Koneti S., Gomes G., Wu K., Lin H. Synthesis and characterization of aluminum oxide–boron carbide coatings by air plasma spraying. Ceramics International. 2010;36(5):1517–1522. https://doi.org/10.1016/j.ceramint.2010.02.024
4. La Salvia J.C., Leavy R.B., Houskamp J.R., Miller H.T., MacKenzie D.E., Campbell J. Ballistic impact damage observations in a hot-pressed boron carbide. In: Advances in ceramic armor V. 2009;45–55. https://doi.org/10.1002/9780470584330.ch5
5. Yamada S., Hirao K., Yamauchi Y., Kanzaki S. B4C–CrB2 composites with improved mechanical properties. Journal of the European Ceramic Society. 2003;23(3):561–565. https://doi.org/10.1016/S0955-2219(02)00094-8
6. Li X., Jiang D., Zhang J., Lin Q., Chen Z., Huang Z. Pressureless sintering of boron carbide with Cr3C2 as sintering additive. Journal of the European Ceramic Society. 2014;34(5):1073–1081. https://doi.org/10.1016/j.jeurceramsoc.2013.11.036
7. Chalgin A.V., Vikhman S.V., Ordan’yan S.S., Danilovich D.P., Nechaeva M.V. Principles of technology and mechanical properties of structural ceramics based on the ternary system SiC–B4C–CrB2. MRS Online Proceedings Library (OPL). 2015;1765:11–16. https://doi.org/10.1557/opl.2015.800
8. Krutskii Yu.L., Nepochatov Yu.K., Pel’ A.N., Skovorodin I.N., Dyukova K.D., Krutskaya T.M., Kuchumova I.D., Mats O.E., Tyurin A.G., Emurlaeva Yu.Yu., Podryabinkin S.I. Synthesis of polydisperse boron carbide and synthesis of a ceramic on its basis. Russian Journal of Applied Chemistry. 2019;92(6):750–758. https://doi.org/10.1134/S1070427219060041
9. Gudyma T.S., Shestakov V.A., Dik D.V., Krutskii Yu.L., Anisimov A.G., Cherkasova N.Yu., Ukhina A.V. Synthesis of B4C/CrB2 powders by boron-carbide reduction using nanofiber carbon for the fabrication of ceramics. Nanobiotechnology Reports. 2023;18:S55–S62. https://doi.org/10.1134/S2635167623600517
10. Gao Y., Etzold A., Munhollon T., Rafaniello W., Haber R. Processing factors influencing the free carbon contents in boron carbide powder by rapid carbothermal reduction. Diamond and Related Materials. 2016;61:14–20. https://doi.org/10.1016/j.diamond.2015.11.005
11. Kovalev I.D., Ponomarev V.I., Vershinnikov V.I., Konovalikhin S.V. SHS-produced boron carbide: Some special features of crystal structure. International Journal of Self-Propagating High-Temperature Synthesis. 2012;21(2):134–138. https://doi.org/10.3103/S1061386212020033
12. Alkan M., Sonmez M.S., Derin B., Yücel O. Effect of initial composition on boron carbide production by SHS process followed by acid leaching. Solid State Sciences. 2012;14(11-12):1688–1691. https://doi.org/10.1016/j.solidstatesciences.2012.07.004
13. Ramos A.S., Taguchi S.P., Ramos E.C.T., Arantes V.L., Ribeiro S. High-energy ball milling of powder B–C mixtures. Materials Science and Engineering: A. 2006;422(1-2): 184–188. https://doi.org/10.1016/j.msea.2006.01.096
14. Podgornyi V.I., Belashev B.Z., Osaulenko R.N., Ternovoi A.N. Synthesis of carbides in the arc plasma. Technical Physics. 2013;(58):1007–1010. https://doi.org/10.1134/S1063784213070165
15. Martynov R.S., Pak А.Ya., Volokitin О.G., Nikitin D.S., Larionov K.B., Povalyaev P.V., Gumovskaya A.A., Bolatova Zh., Vassilyeva Yu.Z. Advanced vacuumless arc plasma synthesis of boron carbide powders and bulk ceramics spark plasma sintering. Bulletin of PNRPU. Mechanical Engineering, Materials Science. 2023;25(3):65–76. (In Russ.). https://doi.org/10.15593/2224-9877/2023.3.07
16. Vassilyeva Yu.Z., Povalyaev P.V., Neklya Yu.A., Pak А.Ya. Modernization of non-vacuum electric arc reactor for boron carbide powder synthesis. Materials. Technologies. Design. 2023;5(5):7–15. (In Russ.). https://doi.org/10.54708/26587572_2023_55157
17. Povalyaev P.V., Pak A.Y., Frantsina E.V., Petrova Y.Y., Egorova V.V. Synthesis of chromium carbide powder by vacuum-free electric arc plasma method. International Journal of Refractory Metals and Hard Materials. 2024;123:106795. https://doi.org/10.1016/j.ijrmhm.2024.106795
18. Povalyaev P.V., Pak A.Y., Nikolaeva K.V., Danilova-Tret’yak S.M. Synthesis of chromium diboride in an arc-discharge atmospheric plasma. Journal of Engineering Physics and Thermophysics. 2024;97:1234–1245. https://doi.org/10.1007/s10891-024-02996-x
19. Andrievski R.A. Micro- and nanosized boron carbide: synthesis, structure and properties. Russian Chemical Reviews. 2012;81(6):549–559. (In Russ.). https://doi.org/10.1070/RC2012v081n06ABEH004287
20. Rutkowski P., Stobierski L., Bućko M.M. The influence of chromium compounds on boron carbide sintering. Composites Theory and Practice. 2013;13(14):245–249.
21. Khasanov O.L., Dvilis E.S., Khasanov A.O., Bikbaeva Z.G., Polisadova V.V., Sokolov V.M., Kachaev A.A., Valova Ya.V. Determination of optimal modes of manufacturing high-density ceramics from boron carbide powder by sintering in spark discharge plasma. Izvestiya Tomskogo Politekhnicheskogo Universiteta. 2012;320(2): 58–62. (In Russ.).
22. Badica P., Grasso S., Borodianska H., Xie S.S., Li P., Tatarko P., Reece M.J., Sakka Y., Vasylkiv O. Tough and dense boron carbide obtained by high-pressure (300 MPa) and low-temperature (1600 °C) spark plasma sintering. Journal of the Ceramic Society of Japan. 2014;122(1424): 271–275. https://doi.org/10.2109/jcersj2.122.271
23. Mahesh B., Sairam K., Sonber J.K., Murthy T.S.R.C., Nageswara Rao G.V.S., Srinivasa Rao T., Chakravartty J.K. Sinterability studies of monolithic chromium diboride (CrB2) by spark plasma sintering. International Journal of Refractory Metals and Hard Materials. 2015;52:66–69. https://doi.org/10.1016/j.ijrmhm.2015.04.035
24. Hirota K., Mitani K., Yoshinaka M., Yamaguchi O. Simultaneous synthesis and consolidation of chromium carbides (Cr3C2 , Cr7C3 and Cr23C6 ) by pulsed electric-current pressure sintering. Materials Science and Engineering: A. 2005;399(1–2):154–160. https://doi.org/10.1016/j.msea.2005.02.062
25. Yamada S., Hirao K., Yamauchi Y., Kanzaki S. Mechanical and electrical properties of B4C–CrB2 ceramics fabricated by liquid phase sintering. Ceramics International. 2003;29(3):299–304. https://doi.org/10.1016/S0272-8842(02)00120-7
About the Authors
Yu. Z. VassilyevaRussian Federation
Yuliya Z. Vassilyeva – Cand. Sci. (Eng.), Research Scientist, Laboratory of Advanced Materials for Energy Industry (LAMEI)
30 Lenin Prosp., Tomsk 634050, Russia
P. V. Povalyaev
Russian Federation
Pavel V. Povalyaev – Junior Research Scientist, LAMEI
30 Lenin Prosp., Tomsk 634050, Russia
A. A. Kuznetsova
Russian Federation
Anastasiya A. Kuznetsova – Student, Weinberg Research Center
30 Lenin Prosp., Tomsk 634050, Russia
A. Ya. Pak
Russian Federation
Aleksandr Ya. Pak – Dr. Sci. (Eng.), Head of the LAMEI
30 Lenin Prosp., Tomsk 634050, Russia
Review
For citations:
Vassilyeva Yu.Z., Povalyaev P.V., Kuznetsova A.A., Pak A.Ya. Obtaining ceramics from boron carbide with chromium-based additives (Cr3C2, CrB2). Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(3):5-14. https://doi.org/10.17073/1997-308X-2025-3-5-14