Scroll to:
SHS of cast materials in the Mo-Al-C system
https://doi.org/10.17073/1997-308X-2023-1-39-48
Abstract
Materials based on molybdenum-aluminium-carbon compounds have a considerable potential for use under intense wear conditions at elevated temperatures. This paper presents the experimental results of self-propagating high-temperature synthesis of compounds within the Mo-Al-C system. By combining two processes: SHS of the elements and SHS-metallurgy, cast materials containing the Mo3Al2C, Mo2C, Mo3Al, and Mo3Al8 phases were obtained. The experiments used mixtures with compositions calculated according to the ratio (1 - α)(3MoO3-8Al-C)/α(3Mo-2Al-C), where a varied in the range from 0 to 1. The synthesis was carried out in a laboratory reactor of 3 L volume at an initial argon pressure of 5 MPa. The mass of the initial mixtures in all experiments was 20 g. The process of combustion was initiated by a 0.5 mm diameter molybdenum wire spiral by applying 28 V voltage to it. The resulting end products were studied by X-ray diffraction and local microstructural analysis. A significant influence of the ratio of the initial reagents on the synthesis parameters, phase composition, and microstructure of the target products was established. Introduction into the high-exothermic mixture 3MoO3-8Al-C inert “cold” mixture 3Mo-2Al-C leads to an increase in the content of carbide phases in the ingots. The possibility of obtaining cast materials based on the triple phase Mo3Al2C, the maximum content of which is 87 wt. % at the content of the “cold” mixture in the charge α = 0.4 is shown. The presence of secondary phases of molybdenum carbide (Mo2C) and molybdenum aluminides (Mo3Al8 , Mo3Al) in the final products is due to a change in the composition of the initial mixture caused by the ejection of components during combustion and insufficient existence time of the melt formed in the combustion wave.
Keywords
For citations:
Gorshkov V.A., Miloserdov P.A., Kovalev D.Yu., Boyarchenko O.D. SHS of cast materials in the Mo-Al-C system. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(1):39-48. https://doi.org/10.17073/1997-308X-2023-1-39-48
Introduction
There are several binary compounds in Mo–Al system – MoAl3 , Mo3Al8 , MoAl, and Mo3Al [1]. These compounds, given their high melting points and mechanical properties, are promising materials for use under high temperature and intense wear conditions [2–4]. In Al–С system, the aluminum carbide phase Al4С3 is stable. Al4С3 particles finely dispersed in the aluminum matrix reduce the creep tendency of the material, especially in combination with silicon carbide particles. Aluminum carbide can be used as an abrasive material in high-speed cutting tools [5; 6]. In the Mo–C system, molybdenum and carbon form carbides Mo2С and МоС1 – х . Among these, molybdenum carbide Mo2C has the most widespread use in practice. It is used as a catalyst in hydrodesulfurization reactions, in dry reforming of methane, for the decomposition of hydrazine, and in regulators of small rocket engines [7–9]. Various methods are used for the synthesis of molybdenum carbide: carbothermal reduction of molybdenum (VI) oxide with graphite in an inert medium, electrochemical synthesis, melting with graphite, reduction of oxide using a methane–hydrogen mixture or other carbon sources [10–12].
Among the ternary molybdenum aluminum carbon compounds, the Mo3Al2C compound, a superconductor with a transition temperature of ~9 K, is of greatest interest. In the studies [13–16], this compound was obtained by the arc and high-frequency melting at high pressures (up to 10 GPa). Its transport, magnetic, and thermodynamic properties were investigated. The bulk modulus of elasticity is estimated at 221 GPa. Known methods for obtaining Mo3Al2C are inefficient and energy-intensive. A one-stage method – self-propagating high-temperature synthesis (SHS) – is a promising method for obtaining such compounds. It requires practically no electricity, exhibits high capacity and is environmentally friendly [17; 18].
The SHS method allowed to obtain a large number of binary and ternary element compounds [19–22]. One of the technological trends of application of self-propagating high-temperature synthesis is SHS-metallurgy, which allows to obtain “cast” materials by the complete melting of components in a combustion wave. A specific feature of the process is the use of mixtures consisting of metal oxides, a reducing metal (Al), and carbon. At a certain ratio of reagents, the combustion temperature exceeds the melting point of the initial reagents and final products. As a result, the product during synthesis is formed in the liquid state. Under the action of gravity, the heavy metal-like and light oxide phases of the formed products are separated. Cast materials based on binary and ternary compounds (MAX phases) in the systems: Cr–Al–C [23–25], Nb–Al–C [26; 27], and V–Al–C [28] obtained by SHS-metallurgy are of great interest. The mentioned studies demonstrate that the main synthesis parameter that determines the composition of the final products is the existence time of the melt, which depends on the combustion temperature of the initial mixture. To date, the authors of this paper have not identified any studies on the production of cast materials in the Mo–Al–C system by the SHS method.
The purpose of this paper was to study the possibility of obtaining cast products within the Mo–Al–C system by combining the methods of SHS from elements and SHS-metallurgy.
Материалы и методика исследований
Powders of MoO3 (“Ch”, purity 99.9 %), Al (ASD-1, purity 99.2 %, particle size d < 30 µm), Mo (PM-M, 99.9 %, d < 10 µm), and graphite (PG, 99.2 %, d < 400 µm) were used as initial reagents. In the experiments, stoichiometric mixtures were used as base mixtures; their composition was calculated from a combination of two chemical reactions:
| 3MoO3 + 8Al + C = Mo3Al2C + 3Al2O3 , | (I) |
| 3Mo + 2Al + C = Mo3Al2C. | (II) |
The mass ratio of the mixtures \(\alpha = \frac{{{M_{{\rm{II}}}}}}{{{M_{\rm{I}}}}}\) varied in the range from 0 to 1, where МI and МII are the masses of the mixtures calculated from reactions (I) and (II), respectively.
The experiments revealed that the combustion of the mixture, the composition of which was calculated according to reaction (I), is characterized by a high rate and a strong ejection of reagents and synthesis products from the reaction vessel. The mixture, the composition of which was calculated according to reaction (II), does not burn and, when added to the first mixture, behaves as a “reactive” inert component. The ratios of the initial compounds in reactions (I) and (II) remained constant in all experiments.
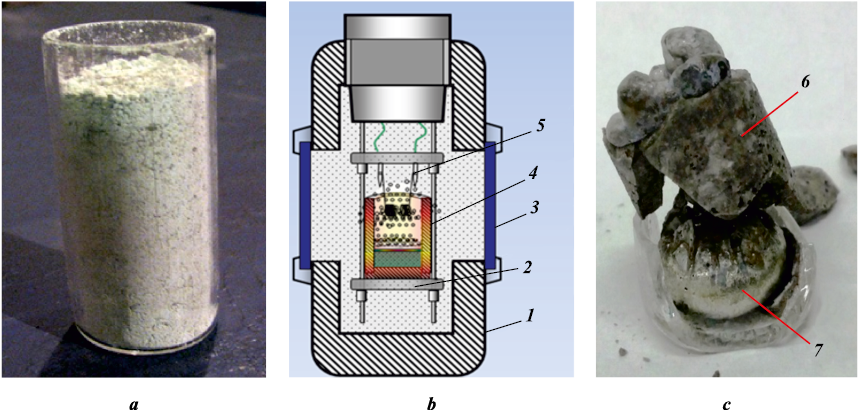
Before mixing, the powders were dried for 3 h at a temperature of 60 °C. The charge was prepared by manually stirring it in a porcelain mortar. The bulk density reaction mixture was placed in a 20 mm in diameter and 50 mm high quartz mold (Fig. 1, a). The weight of the initial mixtures in all experiments was 20 g. The syntheses were carried out in a 3 L reactor (Fig. 1, b) in an argon atmosphere at an initial pressure of 5 MPa according to the procedure described in work [23]. The reaction was initiated with a 0.5 mm diameter molybdenum wire coil. The combustion process was recorded using a video recorder. The combustion rate was calculated from the process video by measuring the time of passage of the combustion wave along the height of the sample. The parameters of synthesis were determined from the following values:
\({\eta _1} = \frac{{{M_{{\rm{ing}}}}}}{{{M_{\rm{0}}}}} \) – the ratio of the product yield in the ingot and the weight of the mixture;
\({\eta _2} = \frac{{{M_{{\rm{ing}}}}}}{{M_{{\rm{ing}}}^{{\rm{calc}}}}}\) – the completeness of the reaction, the ration of the product yield in the ingot relative and its calculated value;
\({\eta _3} = \frac{{{M_0} - {M_{{\rm{ing}}}}}}{{{M_0}}}\) – mass loss during combustion due to the ejection of components from the reaction vessel,
where Ming – the weight of the target product (ingot), \({M_{{\rm{ing}}}^{{\rm{calc}}}}\) – the calculated weight of the ingot, M0 – the mass of the initial mixture, Mk – the total mass of products after burning.
Fig. 1. Quartz crucible with a charge (а), reactor diagram (b), and final products (c) |
The end products of the synthesis were studied using X-ray diffraction (XRD) and microstructural analysis. The methods for studying the obtained products are described in more detail in the authors' previous works [25].
Results and Discussion
The samples after synthesis consist of two ingots that are easily separated mechanically. The material in the upper part of the sample, according to the XRD results, is mainly an α-Al2O3 phase. At the bottom, an ingot (target product) with a characteristic metallic sheen is formed (Fig. 1, c). The formation of an ingot indicates that in the combustion wave a liquid phase was formed, i.e. the synthesis temperature is higher than the melting temperature of the initial components and the resulting products. Different specific masses of the final products lead to their separation due to gravity – the heavy “metal” phase settles at the bottom of the crucible, whereas the light oxide phase forms at the top.
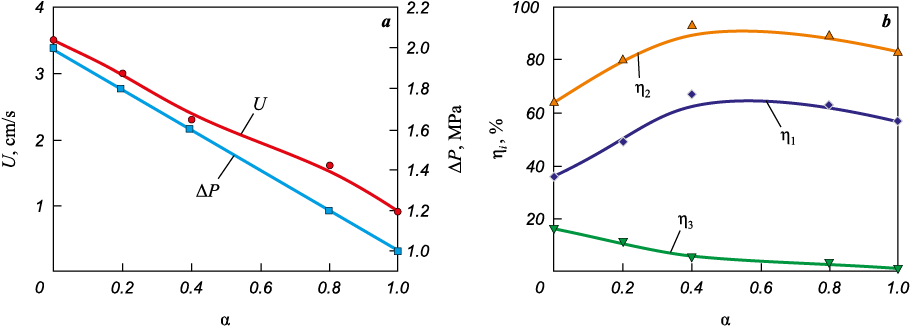
The values of the combustion rate of the initial mixtures and the pressure increase in the reactor depending on the value of α are shown in Fig. 2, a. As α increases, these parameters decrease. When a “cold” mixture, the composition of which is determined from reaction (II), is added to a highly exothermic mixture, the composition of which is determined from reaction (I), part of the heat released as a result of a reaction (I) is spent on its melting, which leads to a decrease in the combustion rate and pressure increase in the reactor. Figure 2, b shows the dependences of the reaction completeness (η2 ), product yield (η1 ), and spread of combustion products (η3 ) on α. The parameters η1 and η2 increase in the range of α from 0 to 0.4, then there is a gradual decrease. The values of η3 decrease monotonically over the entire range of α.
Fig. 2. Influence of α on the combustion rate and pressure increase in the reactor (а) |
The maximum product yield (η1 = 67 %, η2 = 93 %) is observed at α = 0.4 (Fig. 2, b). Yield is affected by two competing factors: reagent spread and combustion temperature. An increase in the amount of the “cold” mixture, on the one hand, reduces the ejection of reagents from the vessel and, accordingly, increases η1 , and, on the other hand, it reduces the combustion temperature, which leads to a decrease in η2 due to a reduction in the time spent by the product in the liquid state, where oxide and “metal” phases spatially separate. X-ray phase analysis of the products (Figs. 3–5) obtained by combustion of mixtures with different α showed that as a result of synthesis, a multiphase material is formed, which includes Mo3Al2C, Mo2C, Mo3Al, and Mo3Al8 . The quantitative ratio of the phases depends on the composition of the initial charge (see Table).
Phase composition of final products (wt. %)
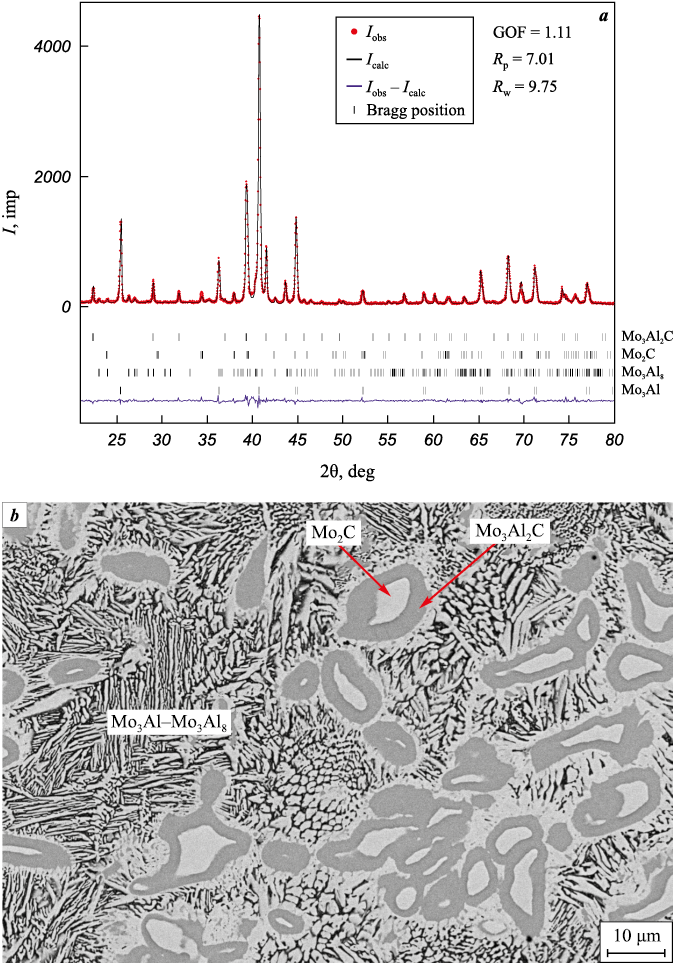
Fig. 3. Diffraction pattern (а) and microstructure (b) of the combustion product of mixture 1 |
The combustion of mixtures of compositions 1 and 2 is non-stationary with a non-linear front and a considerable ejection of material from the crucible. X-ray phase analysis of the obtained products (see Fig. 3) revealed that as a result of synthesis, materials with a high content of molybdenum aluminides (Mo3Al and Mo3Al8 ), more than 65 %, are formed, while the total content of carbide phases (Mo3Al2С and Mo2C) does not exceed 35 %. The low content of the latter is apparently associated with a deficit of carbon due to its ejection from the crucible in the form of particles or gaseous oxides. In the combustion wave, the mixture of initial reagents undergoes a number of physical and chemical transformations. In the heating zone, aluminum and molybdenum oxide melt, forming a liquid-phase medium with distributed carbon particles. In the zone of chemical transformation, aluminum and carbon interact with molybdenum oxide:
| МoО3 + 2Al → Mо + Al2O3 , | (III) |
| МoО3 + хC → MоCх + CO(СО2)↑. | (IV) |
As a result, part of the carbon escapes from the reaction zone in the form of a gas, causing its deficiency in the system. The higher the combustion temperature of the mixture, the greater the likelihood of carbon participation in the redox reaction (IV). The material obtained as a result of the combustion of mixture 1 contains phases of Mo3Al–Mo3Al8 intermetallic compounds forming an eutectoid, as well as Mo3Al2C and Mo2C (see Fig. 3).
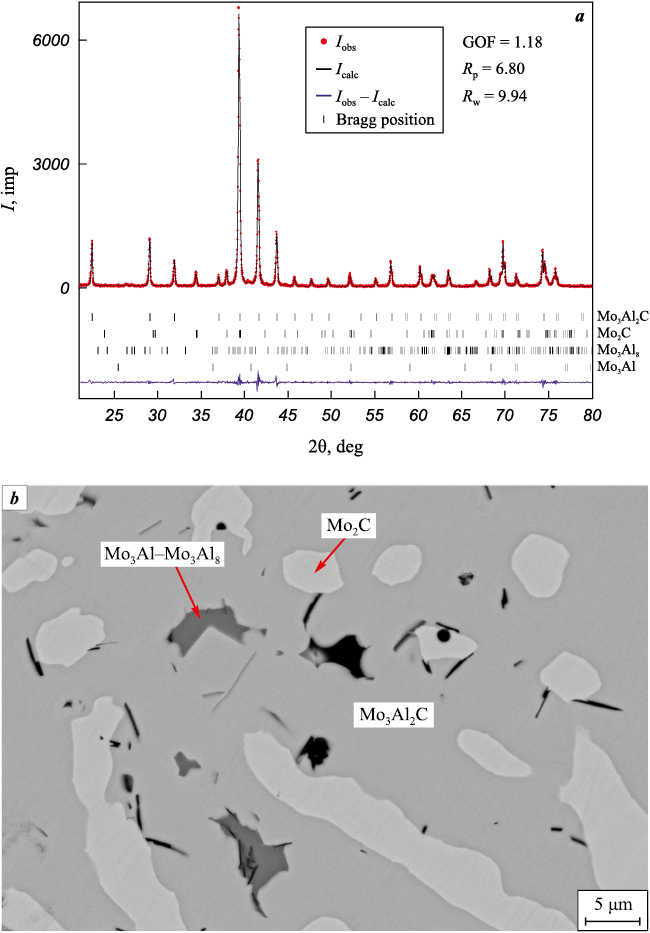
The introduction of a “cold” mixture into the charge leads to an increase of the content of carbide phases in the product while the proportion of molybdenum aluminides decreases (see table). The maximum content (87 %) of the ternary compound Mo3Al2C in the material was obtained at α = 0.4 (see Fig. 4).
Fig. 4. Diffraction pattern (а) and microstructure (b) of the combustion product of a mixture 3 |
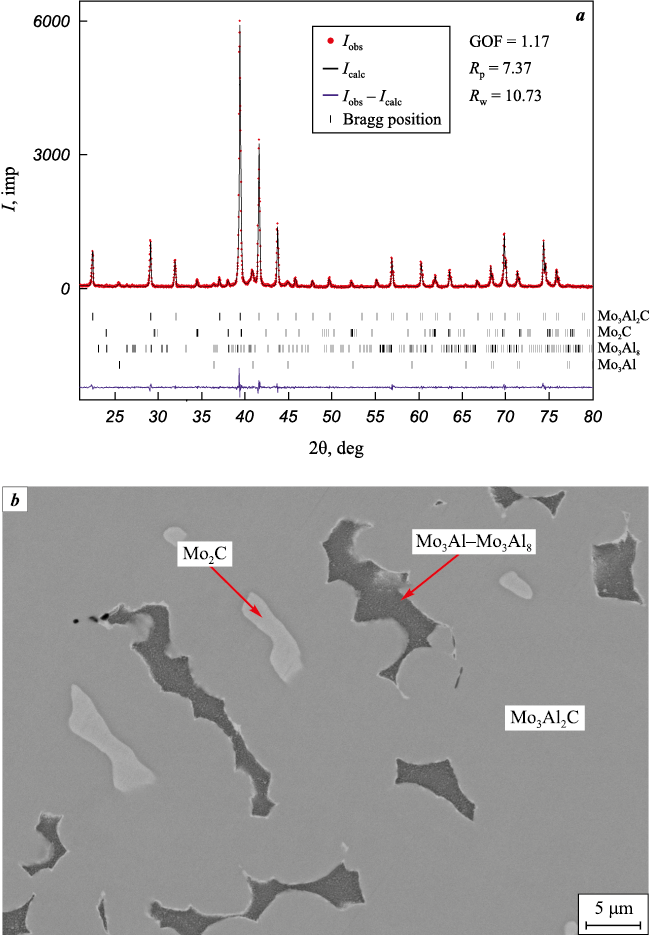
The phase composition of the combustion products of mixture 3 is practically equilibrium and is in the three-phase region Mo3Al2C–Mo2C–Mo3Al. An increase of the “cold” mixture content in the charge over the variation range α = 0.8÷1.0 leads to an increase in the content of Mo2C in the product while reducing the content of Mo3Al2C and molybdenum aluminides (see Table and Fig. 5).
Fig. 5. Diffraction pattern (а) and microstructure (b) of the combustion product of mixture 5 |
The studies show that when a “cold” mixture is added to the charge, the combustion rate, pressure increase, and mass loss (η3 ) decrease over the entire range of α (from 0 to 1.0). At the same time, in the range of α = 0÷0.4, there is an increase in the target product yield into an ingot, whereas at α > 0.4, there is a decrease. The target product yield into the ingot is affected by two competing factors: the content in the charge of the mixture (II), consisting of the target elements (Mo, Al, C) passing into the ingot as compounds, and the combustion temperature. With an increase in the amount of the “cold” mixture, on the one hand, the proportion of elements of the target phase in the charge increases and, accordingly, η1 and η2 increase, and, on the other hand, the combustion temperature decreases, which leads to a decrease in η1 and η2 due to a decrease in the residence time of the product in a liquid state, when there is a spatial separation of the oxide and “metal” phases. The influence of the first factor prevails at α = 0÷0.4, whereas at α > 0.4, prevails the influence of the second factor.
A single-phase product containing only Mo3Al2C in accordance with reactions (I) and (II) was not obtained due to several reasons. Obviously, the above scheme of reactions, used for the calculation of the equilibrium composition of the target ternary phase Mo3Al2C, does not reflect all the interactions actually occurring in the multiphase system during SHS. X-ray diffraction phase analysis of the synthesized material showed that its phase composition differs from the calculated one. This indicates that the processes occurring in the liquid phase formed in the combustion wave and during its rapid crystallization lead to the formation of a nonequilibrium composition of the product. In addition, the combustion process is accompanied by the ejection of components as a result of the reaction (IV). It is quite likely that the resulting material is depleted in both carbon and aluminium. This is indirectly confirmed by the phase composition of mixture 5, which is practically in the two-phase region Mo3Al2C–Mo2C.
The analysis of microstructural analysis data suggests the following mechanism of phase formation of the final product. The phase composition of the ingot is formed as a result of a series of phase transformations. First, refractory Mo2C carbide grains crystallize at a temperature of about 2500 °C. As a result, a Mo–Al melt is formed, which surrounds the Mo2C grains. Then, during cooling in the temperature range of 2500–1720 °C, Al from the melt and Mo2C interact, which leads to the formation of a carbide grain of the Mo3Al2C phase on their surface in a ring pattern (see Fig. 3). The growth of the layer is caused by the diffusion of Al from the Mo–Al melt through the Mo3Al2C layer into the Mo2C grain. At temperatures below 1720 °C, the intergranular melt crystallizes with the formation of Mo3Al and MoAl. Then, at a temperature of 1467 °C, the MoAl phase undergoes the eutectoid transformation MoAl → Mo3Al8 + Mo3Al [1]. Thus, the formation of a multiphase cast material is explained by the multistage nature of its formation and the rapid cooling of the melt.
Conclusion
Cast materials containing the phases Mo3Al2C, Mo2C, Mo3Al, and Mo3Al8 were obtained by the method of self-propagating high-temperature synthesis, combining two modes of the process – SHS from elemental powders and SHS-metallurgy. A significant effect of the ratio of reagents in the initial mixtures on the parameters of the combustion process, microstructure, and phase composition of the products was found. Introduction into the high-exothermic mixture 3MoO3–8Al–C inert “cold” mixture 3Mo–2Al–C leads to an increase in the content of carbide phases in the ingots. The maximum content (~87 wt. %) of the ternary phase Mo3Al2C was obtained at α = 0.4. The presence of carbide Mo2C and molybdenum aluminides Mo3Al8 , and Mo3Al in the final products is due to a change in the stoichiometric composition of the initial charge caused by the ejection of components during the combustion and insufficient existence time of the melt, which leads to the formation of a non-equilibrium composition of the product.
References
1. Диаграммы состояния двойных металлических систем: Справочник. Т. 1. Под ред. Н.П. Лякишева. М.: Машиностроение, 1996. 992 c.
2. Saunders N., Thermotech Ltd. The Al-Mo system (alu-minum-molybdenum). Journal of Phase Equilibria. 1997;18(4):370-378. https://doi.org/10.1007/s11669-997-0063-1
3. Cupid D.M., Fabrichnaya O., Ebrahimi F., Seifert H.J. Thermodynamic assessment of the Al-Mo system and of the Ti-Al-Mo system from 0 to 20 at.% Ti. Intermetallics. 2010;18(6):1185-1196. https://doi.org/10.1016/j.intermet.2010.03.010
4. Kriegel M.J., Walnsch A., Fabrichnaya O., Pavlyuchkov D., Klemm V., Freudenberger J., Rafaja D., Leinewe-ber A. High-temperature phase equilibria with the bcc-type в (AlMo) phase in the binary Al-Mo system. Inter-metallics. 2017;83:29-37. https://doi.org/10.1016/j.intermet.2016.12.004
5. Zhu S.J., Peng L.M., Zhou Q., Ma Z.Y., Kucharova K., Cadek J. Creep behaviour of aluminum strengthened by fine aluminum carbide particles and reinforced by silicon carbide particulates DS Al-SiC/Al4C3 composites. Materials Science and Engineering: A. 1999;268(1-2):236-245. https://doi.org/10.1016/S0921-5093(98)01119-8
6. Gmelins Handbuch Der Anorganischen Chemie. 8th ed. Ed. by the German Chemical Society. System-Num-mer 35: Aluminium. Teil A-Lieferung 1. Pp. iv + 284. Berlin: Verlag Chemie, 1934. https://doi.org/10.1002/jctb.5000541621
7. Wang X.-H., Hao H.-L., Zhang M.-H., Li W., Tao K.-Y. Synthesis and characterization of molybdenum carbides using propane as carbon source. Journal of Solid State Che mistry. 2006;179(2):538-543. https://doi.org/doi:10.1016/j.jssc.2005.11.009
8. Manoli J.-M., Da Costa P., Brun M., Vrinat M., Mauge F., Potvin C. Hydrodesulfurization of 4,6-dimethyldibenzo-thiophene over promoted (Ni,P) alumina-supported molybdenum carbide catalysts: activity and characterization of active sites. Journal of Catalysis. 2004;221(2):365-377. https://doi.org/10.1016/j.jcat.2003.08.011
9. Zeng L., Zhang L., Li W., Zhao S., Lei J., Zhou Z. Molybdenum carbide as anodic catalyst for microbial fuel cell based on Klebsiella pneumoniae. Biosensors and Bioelectronics. 2010;25(12):2696-2700. https://doi.org/10.1016/j.bios.2010.05.002
10. Cho S.J., Lee J., Lee Y.S., Kim D.P. Characterization of iridium catalyst for decomposition of hydrazine hydrate for hydrogen generation. Catalysis Letters. 2006;109(3):181-186. https://doi.org/10.1007/s10562-006-0081-3
11. Anselmi-Tamburini U., Maglia F., Spinolo G., Munir Z.A. Combustion synthesis: An effective tool for the synthesis of advanced materials. Chimica & Industria. 2000;82(10):1-10.
12. Nguyen T.H., Nguyen T.V., Lee Y.J., Safinski T., Adesina A.A. Structural evolution of alumina supported Mo-W carbide nanoparticles synthesized by precipitation from homogeneous solution. Materials Research Bulletin. 2005;40(1):149-157. https://doi.org/10.1016/j.materresbull.2004.09.007
13. Zhu Q., Chen Q., Yang X., Ke D. A new method for the synthesis of molybdenum carbide. Materials Letters. 2007;61(29):5173-5174. https://doi.org/10.1016/j.matlet.2007.04.056
14. Karki A.B., Xiong Y.M., Vekhter I., Browne D., Adams P.W., Young D.P., Thomas K.R., Chan J.Y., Kim H., Prozorov R. Structure and physical properties of the noncentrosymmetric superconductor Mo3Al2C. Physical Review B. 2010;82(6):064512. https://doi.org/10.1103/PhysRevB.82.064512
15. Bonalde I., Kim H., Prozorov R., Rojas C., Rogl P., Bauer E. Evidence for conventional superconducting behavior in noncentrosymmetric Mo3Al2C. Physical Review B. 2011;84(13):134506. https://doi.org/10.1103/PhysRevB.84.134506
16. Sekine C., Sai U., Hayashi J., Kawamura Y., Bauer E. High-pressure synthesis and bulk modulus of non-cent-rosymmetric superconductor Mo3Al2C. Journal of Physics: Conference Series. 2017;950(4):042028. https://doi.org/10.1088/1742-6596/950/4/042028
17. Merzhanov A.G. SHS on the pathway to industrialization. International Journal of Self-Propagating High-Temperature Synthesis. 2001;10(2):237-256.
18. Merzhanov A.G. The chemistry of self-propagating high-temperature synthesis. Journal of Materials Chemistry. 2004;14(12):1779-1786. https://doi.org/10.1039/B401358C
19. Levashov E.A., Mukasyan A.S., Rogachev A.S., Shtan-sky D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. International Materials Reviews. 2017;62(4):203-239. https://doi.org/10.1080/09506608.2016.1243291
20. Lopacinski M., Puszynski J., Lis J. Synthesis of ternary titanium aluminum carbides using self-propagating high-temperature synthesis technique. Journal of the American Ceramic Society. 2004;84(12):3051-3053. https://doi.org/10.1111/j.1151-2916.2001.tb01138.x
21. Zhu C.-C., Zhu J., Wu H., Lin H. Synthesis of Ti3AlC2 by SHS and thermodynamic calculation based on first principles. Rare Metals. 2015;34(2):107-110. https://doi.org/10.1007/s12598-013-0174-2
22. Konovalikhin S.V., Kovalev D.Yu., Sytschev A.E., Vad-chenko S.G., Shchukin A.S. Formation of nanolaminate structures in the Ti-Si-C system: A crystallochemical study. International Journal of Self-Propagating High-Temperature Synthesis. 2014;23(4):217-221. https://doi.org/10.3103/S1061386214040049
23. Gorshkov V.A., Miloserdov P.A., Luginina M.A., Sachkova N.V., Belikova A.F. High-temperature synthesis of a cast material with a maximum content of the MAX phase Cr2AlC. Inorganic Materials. 2017;53(3):271-277. https://doi.org/10.1134/S0020168517030062
24. Gorshkov V.A., Miloserdov P.A., Sachkova N.V., Luginina M.A., Yukhvid V.I. SHS metallurgy of Cr2AlC MAX phase-based cast materials. Russian Journal of Non-Ferrous Metals. 2018;59(5):570-575. https://doi.org/0.3103/S106782121805005X
25. Gorshkov V.A., Miloserdov P.A., Khomenko N.Yu., Sachkova N.V. Production of cast materials based on the Cr2AlC MAX phase by SHS metallurgy using coupled chemical reactions. Russian Journal of Non-Ferrous Metals. 2020;61(3):362-367. https://doi.org/10.3103/S1067821220030086
26. Miloserdov P.A., Gorshkov V.A., Kovalev I.D., Kovalev D.Yu. High-temperature synthesis of cast materials based on Nb2AlC MAX phase. Ceramics International. 2019;45(2, Part A):2689-2691. https://doi.org/10.1016/j.ceramint.2018.10.198
27. Kovalev I.D., Miloserdov P.A., Gorshkov V.A., Kovalev D.Yu. Synthesis of Nb2AlC MAX phase by SHS metallurgy. Russian Journal of Non-Ferrous Metals. 2020;61(1):126-131. https://doi.org/10.3103/S1067821220010083
28. Gorshkov V.A., Karpov A.V., Kovalev D.Yu., Sychev A.E. Synthesis, structure and properties of material based on V2AlC MAX phase. Physics of Metals and Metallography. 2020;121(8)765-771. https://doi.org/10.1134/s0031918x20080037
About the Authors
V. A. GorshkovRussian Federation
Vladimir A. Gorshkov - Dr. Sci. (Eng.), Leading Researcher of the Laboratory of SHS melts and cast materials.
8 Akademican Osip'yan Str., Chernogolovka, Moscow region 142432
P. A. Miloserdov
Russian Federation
Pavel A. Miloserdov - Cand. Sci. (Eng.), Senior Researcher of the Laboratory of SHS melts and cast materials of ISMAN.
8 Akademican Osip'yan Str., Chernogolovka, Moscow region 142432
D. Yu. Kovalev
Russian Federation
Dmitrii Yu. Kovalev - D.Yu. - Dr Sci. (Phys.-Math.), Head of the Laboratory of X-ray investigation of ISMAN.
8 Akademican Osip'yan Str., Chernogolovka, Moscow region 142432
O. D. Boyarchenko
Russian Federation
Ol'ga D. Boyarchenko - Cand. Sci. (Phys.-Math.), Researcher of the Laboratory of materials science of ISMAN.
8 Akademican Osip'yan Str., Chernogolovka, Moscow region 142432
Review
For citations:
Gorshkov V.A., Miloserdov P.A., Kovalev D.Yu., Boyarchenko O.D. SHS of cast materials in the Mo-Al-C system. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2023;17(1):39-48. https://doi.org/10.17073/1997-308X-2023-1-39-48