Scroll to:
Synthesis of (TiTaNb)xHfyZrzC high-entropy carbides resistant to high thermal oxidation by mechanical alloying and spark plasma sintering
https://doi.org/10.17073/1997-308X-2024-1-40-51
Abstract
This study presents the synthesis of (TiZrHfTaNb)C, (TiTaNb)0.45Hf0.275Zr0.275С and (TiTaNb)0.3Hf0.35Zr0.35С single-phase, high-entropy carbides through mechanical alloying and plasma sintering. High-entropy carbides hold promise for applications in jet engine components. We identified optimal mechanical alloying conditions to achieve powder homogeneity and minimize iron fouling. The microstructure, phase, and chemical compositions of the samples were investigated. At 1600 °C, a sample with a face-centered cubic (FCC) lattice and low content of zirconium and hafnium oxides was formed. Elevating the sintering temperature to 2000 °C facilitated oxide dissolution and the formation of single-phase, high-entropy carbides. The microhardness of the samples ranged from 1600 to 2000 HV, while the compressive strength varied between 600 and 800 MPa. Plasma heating tests demonstrated excellent resistance to thermal oxidation for (TiTaNb)0.3Hf0.35Zr0.35С, withstanding temperatures up to 2250 °C.
Keywords
For citations:
Kim A.E., Ozerskoi N.E., Razumov N.G., Volokitina E.V., Popovich A.A. Synthesis of (TiTaNb)xHfyZrzC high-entropy carbides resistant to high thermal oxidation by mechanical alloying and spark plasma sintering. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(1):40-51. https://doi.org/10.17073/1997-308X-2024-1-40-51
Introduction
High Entropy Ceramics (HEC) represent a novel class of materials that has garnered significant interest from the global scientific community. These multicomponent ceramics exhibit superior hardness, wear resistance, and oxidation resistance compared to pure metal carbides [1–9].
Most research focuses on high-entropy carbides containing metals from Group 4 (Ti, Zr, Hf) and Group 5 (V, Nb, Ta) of the Periodic Table. These compounds form monocarbides with a cubic NaCl-type structure, wherein the metals share a common cationic FCC (face-centered cubic) sublattice while carbon occupies the anionic sublattice [10].
To date, numerous high-entropy carbides have been synthesized and investigated. primarily utilizing powder-based methods. The significant distinction lies in the manufacturing processes of high-entropy carbides, with most studies commencing with wet milling and mixing of precursors [4–11].
Metal oxides undergo a carbothermal reaction followed by compaction, spark plasma sintering, or hot isostatic pressing at temperatures ranging from 1600 to 2200 °C. Through this process, (Ti0.2Zr0.2Nb0.2Ta0.2W)C [4] and (Ti0.2Ta0.2Nb0.2Hf0.2W)C [5] were synthesized, along with the high-entropy carbide (CrNbMoWV)С [12]. These powders may contain impurities such as oxides, amorphous carbon, and graphite, indicating potential incompleteness of the carbothermal reaction or variations in carbon content.
The following materials were derived from metal monocarbides: (TiZrNbHfTa)C [13], (HfTaZrNb)C [14], and (Ta0.25Zr0.25Nb0.25Ti0.25)C [10]. However, the resulting ceramic materials exhibit inconsistent phase and chemical compositions and contain inclusions similar to the initial carbides in terms of chemical composition.
In certain studies, elemental metal powders and carbon serve as initial components, following a process similar to the aforementioned works. The following materials were synthesized from elemental powders of metals and carbon: (TiZrHfNbV)C5 [15], (Hf0.2Ta0.2Zr0.2Nb0.2Ti0.2)C and (Hf0.2Ta0.2Ti0.2Mo0.2Nb)C [16], (TiVZrHfNb)C5 , (TiVZrHfTa)C5 , (TiZrNbHfTa)C5 , (TiZrNbVTa)C5 , (TiHfNbVTa)C5 , (ZrHfNbVTa)C5 [17], (Ti0.2Zr0.2Ta0.2Nb0.2W0.2)C [11], (VNbMoTaW)C [18]. However, this process also entails certain drawbacks. Carbides of different metals are formed sequentially, resulting in regions with varying contents of the original elements.
In our papers [19; 20], we demonstrated that spark plasma sintering of CrNbMoWV powder, obtained through mechanical alloying (MA) produces a single-phase coating approximately 100 μm thick on the surface. This coating exhibits enhanced corrosion and wear resistance, surpassing conventional materials in these properties. For instance, the wear rate difference between the carbide layers of a high entropy alloy (0.001 cm3) and WC–8Ni carbide (0.003 cm3) amounts to 300 % [19]. Subsequent research revealed that single-phase, chemically homogeneous high-entropy ceramics can be synthesized from a mixture of high-entropy alloy and carbon MA powders, with the high-entropy carbide (CrNbMoWV)C being the first product of this process [12].
The objective of this study is to explore the production of single-phase, high-entropy ceramic materials with high chemical homogeneity using mechanically alloyed powders of TiZrHfNbTa HEA system and to evaluate their properties.
Materials and methods
We used elemental powders of metals Ti, Nb, Hf, Zr, and Ta (99.5 % purity) as initial components for synthesizing TiZrHfNbTa. Three materials were synthesized: TiZrHfTaNb, (TiTaNb)0.45Hf0.275Zr0.275 and (TiTaNb)0.3Hf0.35Zr0.35 , with their compositions listed in the table. MFG-7 graphite powder served as the carbon source, introduced alongside the elemental metal powders.
Initial alloy compositions after MA
| |||||||||||||||||||||||||||||||||||||
For mechanical alloying, we employed a Pulverisette 4 planetary mill (Fritsch, Germany) under an argon atmosphere. Mechanical alloying occurred over a duration of 5 to 10 h at drive/bowl rpm ranging from 200 to 400. The 500 ml grinding bowls and 12 mm diameter grinding balls were constructed from high-strength carbon steel. Each sample weighed 50 g, and the ball-to-powder weight ratio was maintained at 1:20.
We conducted particle size distribution analysis of the powders using an Analysette 22 NanoTec plus particle sizer (Fritsch, Germany), applying the Fraunhofer diffraction method for calculation.
For sintering, we employed an HPD 25 spark plasma sintering furnace (FCT Systeme GmbH, Germany) with a diameter 20 mm graphite mold, operating at temperatures of 1600, 1800 and 2000 °C, with a pressure of 50 MPa and a holding time of 5 min at the maximum temperature.
For a layer-by-layer examination of phase composition followed plasma heating tests, a SmartLab X-ray diffractometer (Rigaku Corp., Japan) was utilized, employing СuKα radiation (λ = 1.5406 Å) and the CBO-µ Cross Beam Optics system for grazing incidence X-ray diffraction (ω = 10°). The incidence angle range was 20 to 80°, and the acquisition rate was set at 0.2 deg/min. Morphological and microstructural analysis of the powder particles was conducted using a Mira 3 scanning electron microscope (Tescan, Czech Republic). The chemical composition of the powder particles was determined through polished samples and X-ray microanalysis, employing an INCA Wave 500 microanalysis system (Oxford Instruments Analytical, UK) integrated with the scanning electron microscope.
We conducted microhardness measurements using a Buehler microhardness tester (USA) with loads of 300 and 500 g applied to ground and polished samples along the section parallel to the height of the cylindrical sample. Measurements were taken in a straight line with a 330 µm increment from the top to the bottom of the sample. Compressive strength testing was performed using a Zwick/Roell Z050 universal testing machine (Germany).
For plasma heating tests, we employed a UPIM-200 electric arc plasma generator (Composite, Korolev), with plasma generated in the air. Transient heat flow was measured using a cold copper barrier (calorimeter). Initially, the heat flux density was set at 3.1 Mw/m2, and subsequently increased incrementally. The heat flux density increased by 0.4 MW/m2 at each step. Throughout the test, we recorded the surface temperature of the sample using a pyrometer and monitored the surface temperature distribution using a thermal imaging camera.
Results and discussion
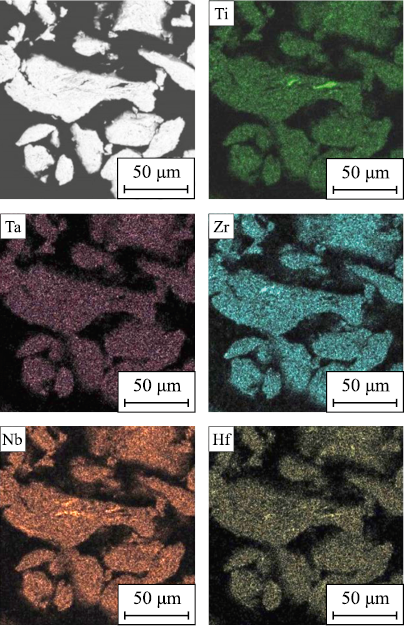
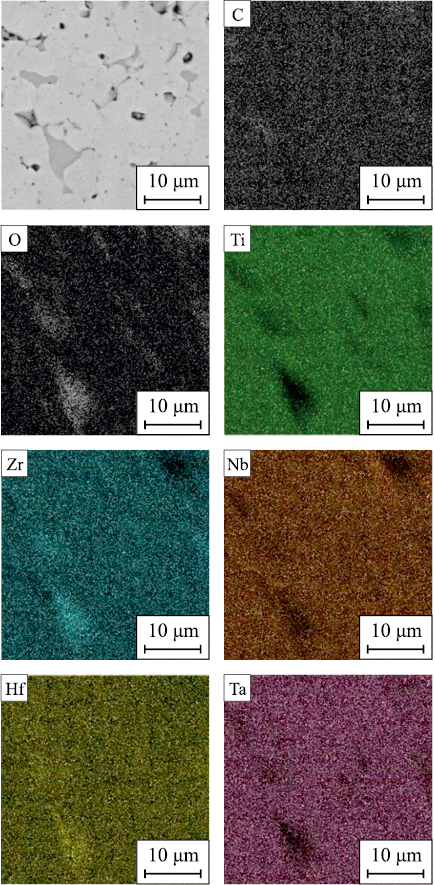
The dissolution of alloying elements in the early stages of mechanical alloying (MA) exhibits similarities across all systems studied. At the onset of MA, intense plastic deformation flattens the initial powder particles, leading to their fusion and the formation of a composite. After 5 h of MA, the composite particles develop a characteristic layered structure consisting of different combinations of the initial components. With further prolongation of MA duration up to 7.5 h, the primary processes involve homogenization of chemical composition and interaction between the initial components aimed at reducing the system’s free energy (Fig. 1).
Fig. 1. Elemental distribution in TiZrHfTaNb alloy powder after τМА = 7.5 h |
When the MA period extends to 10 h, the iron fouling content increases. Specifically, the average iron content in the powder rises from 0.12 % after 7.5 h of MA to 0.59 % after 10 h of MA. The measured particle size distribution (in micrometers, average values) is as follows: at τМA = 5 h – d10 = 19.3, d50 = 47.5, d90 = 87.9; τМA = 7.5 h – d10 = 8.2, d50 = 18.6, d90 = 33.8; τМA = 10 h – d10 = 17.1, d50 = 33.6, d90 = 59.3.
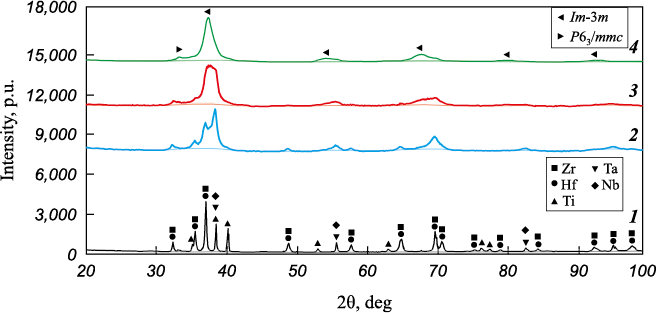
Fig. 2 depicts the XRD patterns of the initial powder mixture and the powder after mechanical alloying for 5, 7.5 and 10 h. The observed peak broadening after MA arises from a reduction in the size of coherent scattering regions and increased micro stresses. After 5 h of MA, titanium is entirely dissolved in the VCCL lattice of niobium and tantalum. This dissolution can be attributed to the similar atomic radii of these elements (Ti = 1.45 Å, Nb = 1.43 Å, Ta = 1.43 Å). However, after 10 h of MA, small peaks of zirconium and hafnium are still present due to the relatively large size of their atoms. The mass fraction of the hexagonal phase (P63 /mmc) is 17 %, with a cubic lattice parameter (Im-3m) is 3.387 Å. The deviation from the cubic lattice parameter calculated by Vegard’s law (a = 3.416 Å) for the equiatomic TiZrHfTaNb material can be attributed to the incomplete dissolution of Hf and Zr.
Fig. 2. Phase composition in TiZrHfTaNb powder during various MA stages |
Based on the analysis of the microstructure, phase composition, elemental distributions, and particle size distributions of the MA samples obtained in the planetary mill, we selected τMA = 7.5 h, with a drive speed of 200 rpm and a bowl speed of 400 rpm, for the synthesis of high-entropy carbides.
High-entropy carbides were then produced from the MA powders using an FCT HPD 25 spark plasma sintering furnace. Fig. 3 displays photographs of 20 mm diameter (TiZrHfTaNb)C samples sintered at 2000 °C after undergoing sandblasting and grinding. Throughout the sintering process, we recorded various process variables including time, temperature, compression stroke, shrinkage rate, current, voltage, power, and compression force.
Fig. 3. Photos of (TiZrHfTaNb)C samples sintered at 2000 °C (20 mm in diameter) |
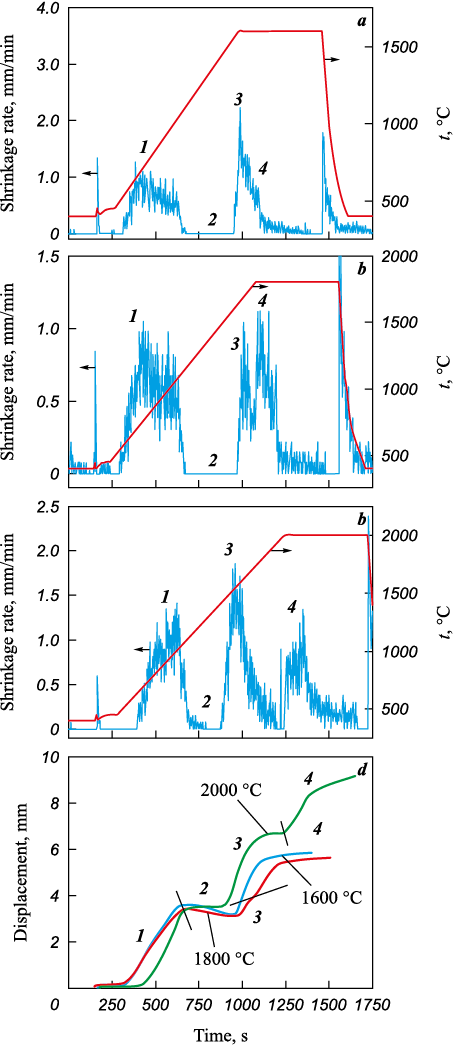
The experimental data obtained during the synthesis of (TiZrHfTaNb)C (Fig. 4) suggest that the process can be divided into four main stages: 1 – degassing and vacuumization; 2 – preheating; 3 – metal-carbon chemical reaction and carbide formation; 4 – compaction under 50 MPa pressure and homogenization for 5 min exposure time. These findings align with temperature profiles utilized in the solid-phase synthesis of TiC, ZrC, HfC, NbC, and TaC metal carbides [13], as well as with data obtained from spark plasma sintering of the (TiZrNbTaW)C alloy using various precursors [11].
Fig. 4. Experimental curves for the synthesis of (TiZrHfTaNb)C |
The microstructure and phase composition of the (TiZrHfTaNb)C samples sintered at 1600 and 1800 °C reveal the presence of high-entropy HCC MeC carbide (Me = Ti, Zr, Hf, Ta, Nb), mixed zirconium-hafnium oxide, and a transition zone from the high-entropy carbide to oxide inclusions. Elevating the sintering temperature of (TiZrHfTaNb)C to 2000 °C results in increased crystal growth capacity (Fig. 5).
Fig. 5. Phase diagrams of (TiZrHfTaNb)C high-entropy carbides sintered at |
The microstructure and elemental distribution of the sample sintered at t = 2000 °C (Fig. 6) indicate the formation of a homogeneous, single-phase, high-entropy carbide. The interface between zirconium-hafnium oxide and high-entropy carbide appears clear, without a zirconium-hafnium-depleted transition zone. The absence of this transition zone and the limited number of oxide inclusions, in comparison to samples sintered at lower temperatures, may suggest the completion of redox reactions and the formation of high-entropy carbides.
Fig. 6. Microstructure and elemental distribution of (TiZrHfTaNb)C sintered at 2000 °C |
From the comparison of the phase composition, microstructure, and chemical homogeneity of the (TiZrHfTaNb)C samples with the thermomechanical data (Fig. 4) obtained during the synthesis, it can be inferred that the stage of chemical interaction and redox reactions (Fig. 4, stage 3) is either completed or nearing completion when the temperature reaches 2000 °C. Stage 4 involves the densification and homogenization of the phase and chemical compositions. The formation of metal carbides is primarily driven by diffusion in the metal and carbon sublattices, with metal and carbon diffusion occurring independently [21; 22]. The diffusion rate of metals is several orders of magnitude lower than that of carbon [23]. As a result, metal diffusion significantly influences the formation of high entropy carbides and restricts the formation of secondary phases. Comparing our results with the synthesis of (TiZrNbTaW)C using a mixture of pure components and a mixture of pure carbides [11] confirms that when the starting material is a high entropy carbide, the kinetics of its formation is linear, resulting in the synthesis product being a single phase (TiZrHfTaNb)C high entropy carbide with high chemical homogeneity.
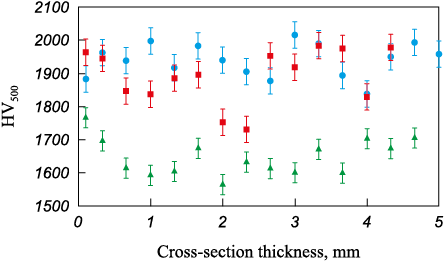
The microhardness measurements (Fig. 7) of the (TiZrHfTaNb)C samples sintered at different temperatures validate the findings from the phase composition and microstructure analysis. The samples sintered at t = 1600 and 1800 °C demonstrate high hardness levels attributed to microscopic stresses induced by the presence of non-equilibrium oxide, carbide, and transition phases. Upon increasing the sintering temperature to 2000 °C, a single-phase, high-entropy carbide is formed. The average hardness values of the samples are 1940, 1917 and 1653 HV, respectively.
Fig. 7. Cross-sectional microhardness of the sintered (TiZrHfTaNb)C samples |
We successfully synthesized (TiTaNb)0.45Hf0.275Zr0.275 and (TiTaNb)0.3Hf0.35Zr0.35 under the same sintering conditions as TiZrHfTaNb)C) (t = 2000 °C). These efforts resulted in obtaining single-phase (TiTaNb)0,45Hf0,275Zr0,275С and (TiTaNb)0,3Hf0,35Zr0,35С carbides.
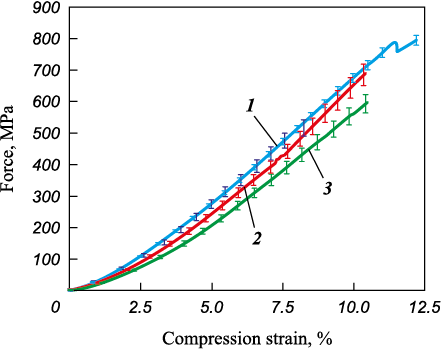
Compression strength tests conducted on the synthesized ceramics (Fig. 8) revealed that the equiatomic (TiZrHfTaNb)C composition exhibited the highest ultimate strength of 795 MPa. As the content of Hf and Zr increased, the ultimate strength decreased to 690 for (TiTaNb)0.45Hf0.275Zr0.275С and 600 MPa for (TiTaNb)0.3Hf0.35Zr0.35С, respectively.
Fig. 8. Compressive strength of samples at room temperature |
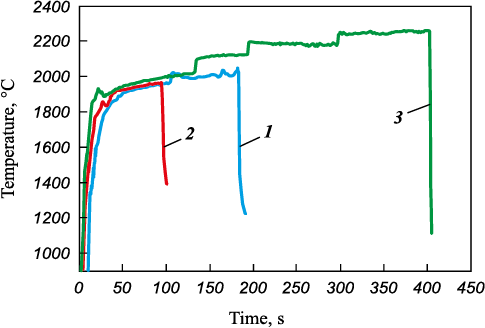
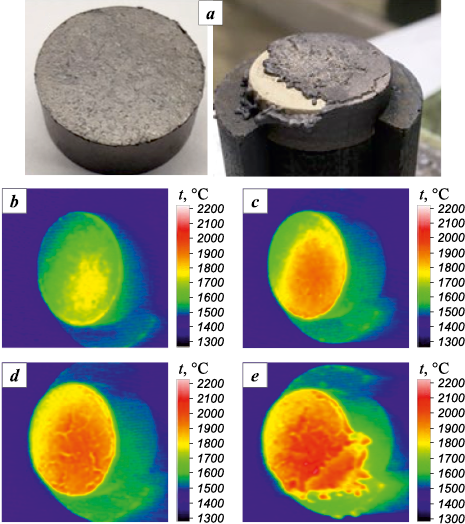
The plasma heating tests conducted on the equiatomic (TiZrHfTaNb)C yielded unsatisfactory results (Fig. 9). Upon heating to t = 1940 °C, the substance melted with intense entrainment of the reaction products and base material.
Fig. 9. Surface temperature vs. duration of plasma heating test |
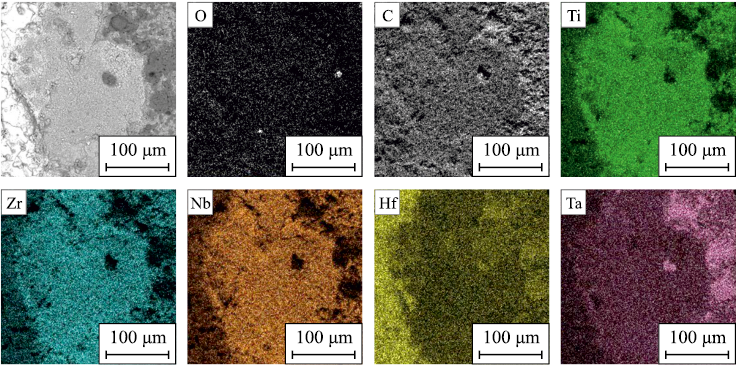
The surface of the (TiZrHfTaNb)C sample following plasma heating tests exhibits regions with varying contents of the initial elements and oxygen (Fig. 10). There are three main types of elemental distribution observed: regions enriched in titanium, zirconium, and niobium; regions enriched in hafnium; and regions enriched in tantalum. The heterophase structure of the oxidation products is attributed to the differing free energies of metal oxide formation at various temperatures. During intense melting, oxidation, and entrainment of reaction products, metals with the lowest free energy of oxide formation are oxidized initially. Consequently, the resulting oxides possess different melting and evaporation temperatures, ultimately influencing the final phase composition of the reaction products.
Fig. 10. Surface morphology and elemental distribution of (TiZrHfTaNb)C sample |
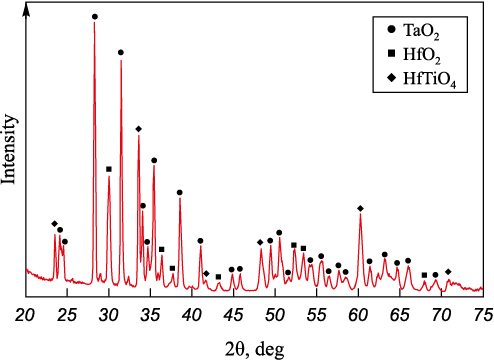
The results of the phase composition analysis of the (TiZrHfTaNb)C sample surface after plasma heating tests (Fig. 11) validate the formation of three main phases identified through elemental distribution analysis. The hafnium-enriched phase corresponds to a mixed metal dioxide exhibiting a monoclinic lattice structure (HfO2 prototype). The second phase, enriched in titanium, zirconium, and niobium, is characterized by the presence of mixed oxide (TiNbZr)O2 with an orthorhombic lattice. This phase formation is attributed to high supercooling rates during the process, preventing the separation of the mixed oxide based on the higher niobium oxide Nb2O5 . A similar appearance of this phase was observed during plasma heating tests of equiatomic high entropy carbide with a comparable composition. The third phase identified is the unstable TaO2 oxide featuring a cubic lattice structure.
Fig. 11. XRD pattern of the oxidation products on the surface |
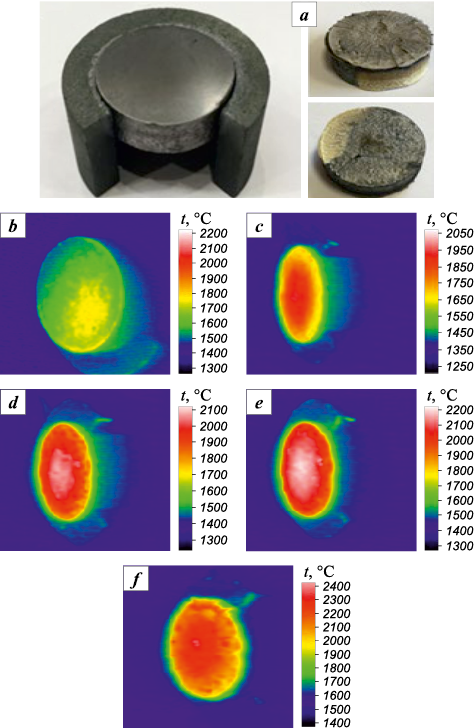
During testing, the (TiTaNb)0.45Hf0.275Zr0.275C and (TiTaNb)0.3Hf0.35Zr0.35C samples were subjected to an initial heat flux density of 3.1 MW/m2, which was increased by 0.4 MW/m2 at subsequent stages. The appearance of the samples before and after testing is illustrated in Figs. 12, a and 13, a respectively.
Fig. 12. (TiTaNb)0.45Hf0.275Zr0.275C sample before/after plasma heating tests (a)
Fig. 13. (TiTaNb)0.3Hf0.35Zr0.35C sample before/after plasma heating tests (a) |
In the case of the (TiTaNb)0.45Hf0.275Zr0.275C sample, the transition to 3.9 MW/m2 (τ = 170 s) resulted in burst boiling and subsequent destruction, as depicted in Fig. 12. The test was terminated after a cumulative time of 175 s, with the maximum temperature reached before sample failure being 2000 °С.
The (TiTaNb)0.3Hf0.35Zr0.35C sample underwent testing for 400 s. This demonstrated high resistance to thermal oxidation, with the surface temperature reaching 2250 °C. Subsequently, a liquid phase appeared (Fig. 13).
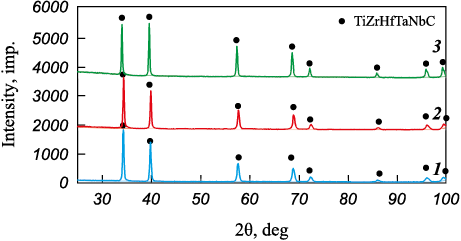
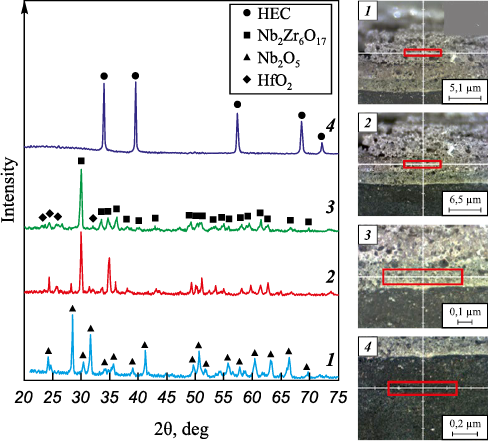
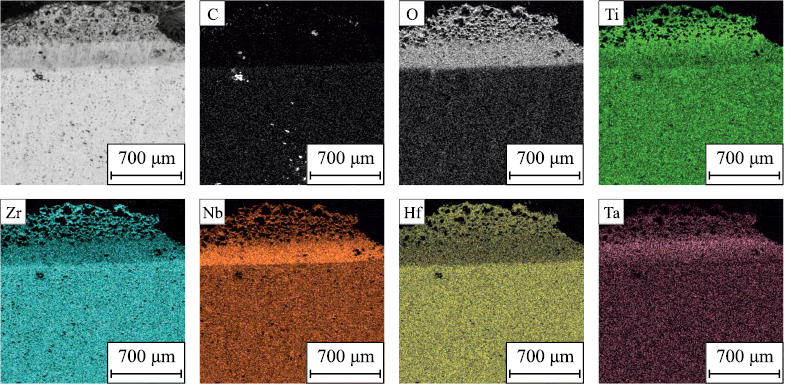
The layer-by-layer phase analysis of the oxidation products of the (TiTaNb)0.45Hf0.275Zr0.275C and (TiTaNb)0.3Hf0.35Zr0.35C samples revealed the segregation of layers based on the phase components (Figs. 14–16). On the surface, MeO2 (a precursor of ZrO2 with tmelt = 2715 °C); a high-melting mixed dioxide, was formed as the first layer. The second layer predominantly consists of Me2Me6O17 (a precursor of Nb2Zr6O17 with tmelt = 1670 °C); a low-melting mixed oxide. The third layer comprises Me2O5 (a precursor of Nb2O5 with tmelt = 1512 °C), another low-melting mixed oxide. The analysis confirms the formation of layers enriched in Zr and Hf, as well as Nb and Ta.
Fig. 14. Layer-by-layer phase composition analysis of (TiTaNb)0.3Hf0.35Zr0.35C sample cross-section
Fig. 15. Elemental distribution along the cross-section of (TiTaNb)0.3Hf0.35Zr0.35C sample
Fig. 16. Elemental content in the reaction products (1), |
It is noteworthy that the (TiTaNb)0.3Hf0.35Zr0.35C sample exhibited high resistance to thermal oxidation, which can be attributed to the formation of a high-melting oxide layer.
Conclusion
In summary, we have successfully synthesized single-phase, high-entropy (TiZrHfTaNb)C ceramic materials with high chemical homogeneity through mechanical alloying. This homogeneity results from the mixing of metals at the atomic level, leading to the formation of a single-phase solid solution.
Our investigation revealed that the optimal MA period is 10 h, irrespective of the initial composition. During MA, a solid solution with a face-centered cubic (FCC) lattice structure is formed, a process occurring in stages dependent on the atomic radii of the initial components. Specifically, elements with smaller atomic radii, such as Nb (0.145 nm), Ti (0.146 nm), and Ta (0.146 nm), dissolve first, followed by Hf (0.159 nm) and Zr (0.160 nm).
We have developed a process for obtaining single-phase, multicomponent ceramic materials from mechanically alloyed TiZrHfTaNb. Spark plasma sintering was utilized to investigate the physical and chemical properties of these materials. Through this process, we have successfully fabricated single-phase, equiatomic, and modified high-entropy carbides based on TiZrHfTaNb, which demonstrate resistance to high-temperature oxidation.
Furthermore, plasma heating tests revealed that resistance to thermal oxidation increases with higher Hf and Zr content. The equiatomic high-entropy carbide exhibited satisfactory results at 1900 °C. Additionally, the (TiTaNb)0.3Hf0.35Zr0.35C high-entropy ceramic material endured testing for 400 s at a surface temperature of 2250 °С.
References
1. Kan W.H., Zhang Y., Tang X., Lucey T., Proust G., Gan Y., Cairney J. Precipitation of (Ti, Zr, Nb, Ta, Hf)C high entropy carbides in a steel matrix. Materialia. 2019;1;100540. https://doi.org/10.1016/j.mtla.2019.100540
2. Braic V., Vladescu A., Balaceanu M., Luculescu C.R., Braic M. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surface Coatings Technology. 2012;211:117–121. https://doi.org/10.1016/j.surfcoat.2011.09.033
3. Murty B.S., Yeh J.W., S. Ranganathan P.P.B. High-entropy alloys. Advanced in Materials Science and Engineering. 2015;1:1.
4. Zhang Y., Zhou Y.J., Lin J.P., Chen G.L., Liaw P.K. Solid-solution phase formation rules for multi-component alloys. Advanced Engineering Materials. 2008;10(6):534–538. https://doi.org/10.1016/j.mtla.2019.100540
5. Guo S., Ng C., Lu J., Liu C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. Journal of Applied Physics. 2011;109:103505. https://doi.org/10.1063/1.3587228
6. Zhang Y., Yang X., Liaw P.K. Alloy design and properties optimization of high-entropy alloys. JOM. 2012;64: 830–838. https://doi.org/10.1007/s11837-012-0366-5
7. Wang K., Chen L., Xu C., Zhang W., Liu Z., Wang Y., Zhou Y. Microstructure and mechanical properties of (TiZrNbTaMo)C high-entropy ceramic. Journal of Materials Science and Technology. 2020;39:99–105. https://doi.org/10.1016/j.jmst.2019.07.056
8. Yang Y., Wang W., Gan G.-Y., Shi X.-F., Tang B.-Y. Structural, mechanical and electronic properties of (TaNbHfTiZr)C high entropy carbide under pressure: Ab initio investigation. Physica B: Condensed Matterials. 2018;550:163–170. https://doi.org/10.1016/j.physb.2018.09.014
9. Zhang H., Hedman D., Feng P., Han G., Akhtar F. A high-entropy B4(HfMo2TaTi)C and SiC ceramic composite. Dalton Transactions. 2019;48:5161–5167. https://doi.org/10.1039/c8dt04555k
10. Wang F., Yan X., Wang T., Wu Y., Shao L., Nastasi M., Cui B. Irradiation damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C high-entropy carbide ceramics. Acta Materialia. 2020;195: 739–749. https://doi.org/10.1016/j.actamat.2020.06.011
11. Wei X.-F., Liu J.-X., Li F., Qin Y., Liang Y.-C., Zhang G.-J. High entropy carbide ceramics from different starting materials. Journal of the European Ceramic Society. 2019;39:2989–2994. https://doi.org/10.1016/j.jeurceramsoc.2019.04.006
12. Wei X.-F., Qin Y., Liu J.-X., Li F., Liang Y.-C., Zhang G.-J. Gradient microstructure development and grain growth inhibition in high-entropy carbide ceramics prepared by reactive spark plasma sintering. Journal of the European Ceramic Society. 2020;40:935–941. https://doi.org/10.1016/j.jeurceramsoc.2019.12.034
13. Zhou J., Zhang J., Zhang F., Niu B., Lei L., Wang W. High-entropy carbide: A novel class of multicomponent ceramics. Ceramics International. 2018;44:22014–22018. https://doi.org/10.1016/j.ceramint.2018.08.100
14. Csanádi T., Vojtko M., Dankházi Z., Reece M.J., Dusza J. Small scale fracture and strength of high-entropy carbide grains during microcantilever bending experiments. Journal of the European Ceramic Society. 2020;40:4774–4782. https://doi.org/10.1016/j.jeurceramsoc.2020.04.023
15. Chicardi E., García-Garrido C., Gotor F.J. Low temperature synthesis of an equiatomic (TiZrHfVNb)C5 high entropy carbide by a mechanically-induced carbon diffusion route. Ceramics International. 2019;45(17):21858–21863. https://doi.org/10.1016/j.ceramint.2019.07.195
16. Moskovskikh D.O., Vorotilo S., Sedegov A.S., Kuskov K.V., Bardasova K.V., Kiryukhantsev-Korneev P.V., Mukasyan A.S. High-entropy (HfTaTiNbZr)C and (HfTaTiNbMo)C carbides fabricated through reactive high-energy ball milling and spark plasma sintering. Ceramics International. 2020;46:19008–19014. https://doi.org/10.1016/j.ceramint.2020.04.230
17. Chicardi E., García-Garrido C., Hernández-Saz J., Gotor F.J. Synthesis of all equiatomic five-transition metals high entropy carbides of the IVB (Ti, Zr, Hf) and VB (V, Nb, Ta) groups by a low temperature route. Ceramics International. 2020;46:21421–21430. https://doi.org/10.1016/j.ceramint.2020.05.240
18. Liu D., Zhang A., Jia J., Meng J., Su, B. Phase evolution and properties of (VNbTaMoW)C high entropy carbide prepared by reaction synthesis. Journal of the European Ceramic Society. 2020;40:2746–2751. https://doi.org/10.1016/j.jeurceramsoc.2020.03.020
19. Razumov N., Makhmutov T., Kim A., Shemyakinsky B., Shakhmatov A., Popovich V., Popovich A. Refractory crmonbwv high-entropy alloy manufactured by mechanical alloying and spark plasma sintering: Evolution of microstructure and properties. Materials (Basel). 2021;14: 1–14. https://doi.org/10.3390/ma14030621
20. Makhmutov T., Razumov N., Popovich A. Synthesis of single-phase high-entropy carbides from a mixture of pre-mechanically alloyed CrNbMoWV HEA powders and carbon. Materials Letters. 2022;309:131363. https://doi.org/10.1016/j.matlet.2021.131363
21. Yut B.B. Nb G. of 14C in single crystals. Crystals. 1979;40:997–1006.
22. Zhang H., Akhtar F. Processing and characterization of refractory quaternary and quinary high-entropy carbide composite. Entropy. 2019;21(5):474. https://doi.org/10.3390/e21050474
23. Demaske B.J., Chernatynskiy A., Phillpot S.R. First-principles investigation of intrinsic defects and self-diffusion in ordered phases of V2C. Journal of Physics: Condensed Matterials. 2017;29(24):245403. https://doi.org/10.1088/1361-648X/aa7031
About the Authors
A. E. KimRussian Federation
Artem E. Kim – Engineer of the Laboratory “Synthesis of new materials and structures”
29 Polytekhnicheskaya Str., St. Petersburg 195251, Russian Federation
N. E. Ozerskoi
Russian Federation
Nikolai E. Ozerskoi – Research Associate of the Scientific and Educational Centre “Additive Technologies”
29 Polytekhnicheskaya Str., St. Petersburg 195251, Russian Federation
N. G. Razumov
Russian Federation
Nilkolai G. Razumov – Cand. Sci. (Eng.), Head of Laboratory “Synthesis of new materials and structures”
29 Polytekhnicheskaya Str., St. Petersburg 195251, Russian Federation
E. V. Volokitina
Russian Federation
Ekaterina V. Volokitina – Engineer of the Russian-Chinese Research Laboratory “Functional Materials”
29 Polytekhnicheskaya Str., St. Petersburg 195251, Russian Federation
A. A. Popovich
Russian Federation
Anatoly A. Popovich – Dr. Sci. (Eng.), Professor, Director of the Institute of Machinery, Materials and Transport
29 Polytekhnicheskaya Str., St. Petersburg 195251, Russian Federation
Review
For citations:
Kim A.E., Ozerskoi N.E., Razumov N.G., Volokitina E.V., Popovich A.A. Synthesis of (TiTaNb)xHfyZrzC high-entropy carbides resistant to high thermal oxidation by mechanical alloying and spark plasma sintering. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(1):40-51. https://doi.org/10.17073/1997-308X-2024-1-40-51