Scroll to:
Influence of copper salts on the physical and mechanical properties of copper–graphite composite materials
https://doi.org/10.17073/1997-308X-2024-2-5-13
Abstract
We investigated composite materials based on electrolytic copper powder containing 1 and 5 wt. % powder of colloidal graphite the addition of trace amounts of copper sulfate and acetate. The materials were obtained through double cold pressing in a mold at a pressure of 600 MPa, intermediate sintering (annealing) in hydrogen at a temperature of 870 °C, and final sintering in vacuum at the copper premelting temperature. To analyze the influence of copper salts on the density, porosity, electrical resistivity, and strength of copper–graphite composite materials, we employed X-ray phase analysis, scanning electron microscopy, conducted strength tests in three-point bending, and determined electrical resistivity. We established that higher graphite content results in increased porosity and electrical resistivity of composite materials, along with decreased strength. In the materials containing copper sulfate, copper is reduced from the salt in the form of nanodispersed particles on the surfaces and inside graphite flakes, leading to a decrease in electrical resistivity compared to copper–graphite composites without salt additives. When copper acetate was added to the composite material, copper is reduced from the salt mainly on the surfaces of graphite particles in the form of microdispersed particles and their aggregations, as the copper acetate solution does not wet the graphite. In this case, the electrical resistivity was somewhat higher than that of the composite with sulfate but lower than that of the material without salts. The bending strength of the studied materials decreased as salts were introduced due to increased porosity and emerging defects in the crystal structure of graphite during its intercalation with copper.
Keywords
For citations:
Ogleznev N.D., Yakubaev I.I., Oglezneva S.А., Porozova S.Е. Influence of copper salts on the physical and mechanical properties of copper–graphite composite materials. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(2):5-13. https://doi.org/10.17073/1997-308X-2024-2-5-13
Introduction
Copper–carbon composites combine the high thermal and electrical conductivity of copper alongside the low thermal expansion coefficient, specific mass, and high melting point of carbon [1]. Consequently, they find extensive application in electrical devices and the semiconductor industry, serving as materials for thermally conductive bases in the housings of high-power rectifying and laser diodes, microwave transistors, power amplifiers [2; 3], as well as in electro-erosion machining of metals, heavy-duty power modules, and optoelectronic devices such as pantograph sliders, brushes in electric motors, and other machinery components [4]. However, the electrical resistivity of graphite is two orders of magnitude greater than that of copper, leading to a decrease in the overall electrical conductivity of the composite material (CM) when graphite is added in large quantities.
Intercalation with metal ions, including the formation of superconducting structures, can enhance the graphite conductive properties. For example, graphite intercalated with calcium exhibits superconductivity [5], while iron enhances the thermal and electrical conductivity of carbon materials [6]. Copper intercalation into graphite [7; 8] and carbon nanotubes (CNTs) [9] has also been reported. However, the lack of physicochemical interaction between the components, including the high surface tension of the metal melt, poses challenges to the manufacturing technologies for copper–graphite and copper–CNT systems [10]. Metal ions are introduced into graphite, CNTs, and fullerenes using salt solutions. For instance, in [9], CNT powder was mixed with copper acetate hydrate, and quantum copper wires up to 50 nm long were obtained after thermal treatment inside CNTs. The authors in [7; 8] utilized copper chloride for graphite intercalation to produce superconducting materials [11].
Due to the significant difference in thermal expansion coefficients, conventional preparation methods struggle to achieve effective interfacial adhesion between the copper matrix and carbon. Even rolling at different temperatures with high degrees of deformation [13] fails to strengthen the phase interface and reduce electrical resistivity.
Since graphite lacks physicochemical interaction with the copper matrix, carbide-forming elements such as boron, chromium, etc. [14] are added to the CM to enhance bonding between copper and carbon at the phase interface. Additionally, the carbon surface is oxidized with acids [15] and salts, resulting in the introduction of ultra-dispersed copper into the material pores. This process leads to reduced friction coefficient and wear, while improving electrical conductivity and mechanical properties [16]. In [17], preliminary chemical copper-plating of natural flake graphite is utilized to enhance the adhesion of graphite to the copper matrix, albeit complicating the technological process and increasing costs.
There are documented instances of obtaining intercalated structures of graphite without special processing. In [18], graphite was introduced into a copper melt at 1200–1250 °C, yielding a copper alloy with lower electrical resistivity and higher tensile strength compared to existing ones. Authors in [19] conducted intercalation by incubating samples of highly oriented pyrolytic graphite (HOPG) for 20 min in a 99.99 % pure copper melt at 1473 K in vacuum. Analysis of the diffraction pattern of graphite containing copper atoms revealed planes with atoms displaced from their initial positions within its structure. The authors explain these results by the formation of intermediate complexes with copper ions in graphite. If the metal atom leaves a pair of rings in the plane of the graphite grid, the latter instantly establishes “diamond” bonds with molecular networks corrugating in the area where the metal was located.
The powder metallurgical technique enables to control the properties of composite materials by adjusting compositions and manufacturing methods across a wide range of options. In [20], a copper–graphite composite material was fabricated through sintering at a pre-melting copper temperature. Additional reflections identified via X-ray phase analysis of the sintered CM of copper and colloidal graphite corresponded to those described in [21]. The resulting materials exhibited low electrical resistivity and were evaluated as electrodes-tools for electro-erosion machining, benefitting from their high electrical and thermal conductivity properties.
Considering the method of pretreating graphite with acids and subjecting it to high pressure of copper vapor, the formation of intercalated compounds with copper appears quite feasible [21; 22].
The objective of the current study is to investigate the impact of treating graphite with copper salts on the physical and mechanical properties of copper–graphite CMs.
Experimental and research techniques
To produce copper–graphite samples, we utilized PMS-1 copper powder (according to GOST 4960-75), S-1 colloidal graphite (per TS 113-08-48-63-90), CuSO4 (according to GOST 19347–2014), or (CH3COO)2Cu salt, which was prepared from copper powder and glacial acetic acid. The surface of compacted graphite powder was moistened with 7 %-aqueous solutions of salts using the sessile drop method, with the wetting angle being determined based on photographs. In some instances, non-ionogenic surfactants were added to the salt solutions.
We mixed S-1 graphite powder with copper salts in amounts sufficient to achieve 10 % copper content after reduction. Distilled water with or without the required amounts of salts and surfactants was added, and the powder was dispersed in an ultrasonic bath (USB) ST-400S (Russia), then dried at room temperature and subsequently reduced in hydrogen at temperatures ranging from 750 to 1000 °C. For the production of composite materials (CMs), copper and graphite powders were mixed in proportions of 1 or 5 wt. % with a non-ionogenic surfactant (with salts added in some cases). The mixtures were dispersed in the USB (ST-400S) with ethyl alcohol and dried. The resulting powders were pressed at a pressure of 600 MPa using the P-125 press (manufactured in Russia) and annealed in the SGV furnace (manufactured in Russia) in a hydrogen atmosphere at 870 °C for 1 h. After annealing, samples were additionally compacted in the mold at a pressure of 600 MPa. Finally, the samples were sintered in a vacuum at temperatures ranging from 1070 to 1080 °C for 2 h using the SNVE-1.3.1/16 furnace (Russia).
The X-ray phase analysis was conducted using the XRD-6000 diffractometer (Shimadzu, Japan) with CuKα-radiation. The phase composition was identified using the International Center for Diffractographic Measurements files, and Crystallographica Search-Match Version 2.0.3.1 (Oxford Cryosystems Ltd), was employed for data processing. The shooting parameters included an angle range from 10 to 110° with a step of 0.02°. The structure of copper–graphite CM samples was examined using a Tescan Vega 3 scanning electron microscope equipped with an EDX-analyzer (Czech Republic).
The properties were tested on 3–10 samples per point. The density and porosity of composite materials were determined using the calculation method according to the standard technique (GOST 18898–89). The electrical resistivity of the CM was calculated from the sample resistance determined by the GOM-802 device (Russia), using a method based on measuring the potential difference across the conductor section. The strength at three-point bending of samples without cracks was tested using the FP 10/1 machine (Germany), following the procedures outlined in GOST 18227–85, with a loading speed of 2 mm/min and a distance of 40 mm between the supports.
Results and discussion
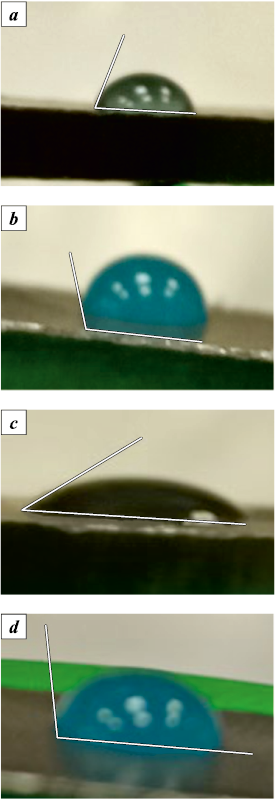
The contact angle of wetting the graphite surface with copper sulfate solution was significantly smaller than that of wetting it with copper acetate solution (Fig. 1, a, b). Upon addition of a non-ionogenic surfactant to the aqueous solutions of both salts, these values decreased further: from 70 to 34° for copper sulfate (Fig. 1, c), indicating wetting according to the well-known Thomas Young formula, suggesting that the solution with surfactant is close to spreading on graphite. However, for copper acetate, the contact angle decreased only insignificantly, from 110 to 98°, implying nonwettability (Fig. 1, d).
Fig. 1. Determination of the contact angle of wetting |
It is noteworthy that even after several minutes, drops of salt solutions continued to spread on the graphite surface, indicating its interaction with the salts. The experiment involving the reduction of graphite powder treated with salt solutions allowed us to simulate the mechanism of forming the structure of copper–graphite composite material during sintering.
We examined the phase composition after the reduction of copper salts in mixtures with graphite in hydrogen (Table 1). It was observed that copper acetate is reduced to pure copper even at a temperature of 750 °C, with a small amount of Cu2O oxidized copper formed (Table 1). As the temperature increases to 1000 °C, copper oxide is no longer detected, which is consistent with previous data on copper reduction in hydrogen at temperatures ranging from 200 to 400 °C [23]. At 750 °C, copper sulfate transforms into copper sulfide, and as the temperature rises to 1000 °C, the sulfide is reduced to copper, in accordance with thermodynamic calculations [24].
Table 1. Phase composition of graphite impregnated with salt after reduction
| |||||||||||||||||||||||||
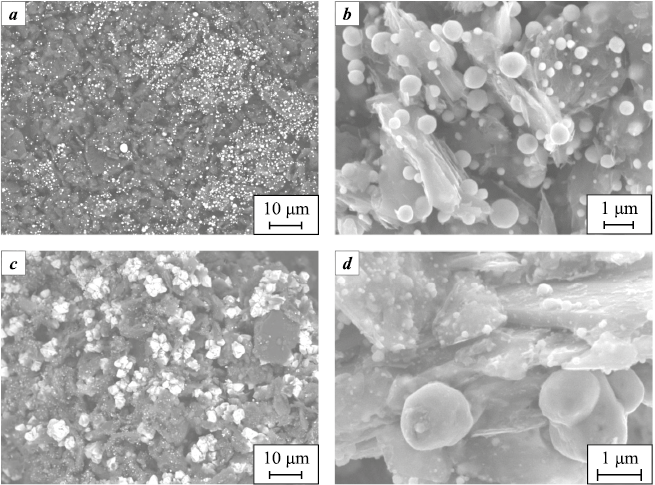
SEM images of graphite mixtures after reduction with copper salts reveal that in the sample treated with copper sulfate, copper particles are distributed both on the surfaces and inside the graphite particles and the distribution is quite uniform (Fig. 2, а, b). Surface particles account for approximately 20 %, with a maximum of 50 % (Fig. 2, a, Table 1). The copper particles reduced from sulfate measure between 0.2 and 1.0 μm in size (Fig. 2, b, Table 1).
Fig. 2. SEM image of S-1 graphite powder after impregnation |
In the graphite sample treated with an aqueous copper acetate solution and impregnated to a shallow depth, the reduced copper particles are predominantly located on the surfaces of graphite particles (Fig. 2, c, d) in the form of large crystals, with a concentration on the surface reaching 60 % (78 % maximum, Table 1). The particles of copper reduced from acetate range from 0.1 to 3.0 μm in size. Given that the average copper content in both samples was approximately 10 wt. % relative to the graphite mass, it is evident that the majority of copper reduced from acetate is concentrated on the surfaces of graphite particles, while in the sample treated with copper sulfate, copper particles are mostly situated in the interlayer spaces of graphite flakes. These results suggest a similar reduction of copper from salts during CM sintering after their addition.
After the final sintering of composite materials containing 99–95 % of PMS-1 copper powder and 1–5 % of colloidal graphite powder (with and without addition of salts), SEM images reveal that copper, in the form of spheres of varying diameters, is uniformly distributed within graphite inclusions (Fig. 3–5).
On the fracture surface of the sintered CM sample with 5 % graphite without the addition of salts (Fig. 3, a), dispersed copper particles ranging in size from 0.1 to 0.5 µm are observed in small concentrations. These particles are located both inside and on the surfaces of graphite particles (Fig. 3, b).
Fig. 3. SEM images of a copper-based CM sample |
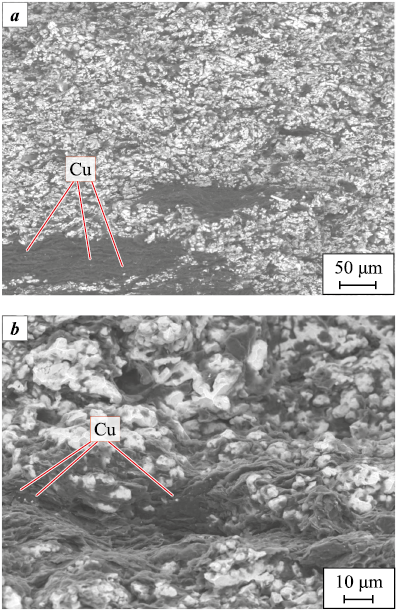
In the SEM images of the sintered sample containing 1 % graphite with the addition of copper sulfate (Fig. 4), copper particles measuring 5–10 μm in size are uniformly distributed in large quantities, appearing on both the surfaces of graphite flakes and between the layers of particles (Fig. 4, b).
Fig. 4. SEM images of a copper-based CM sample |
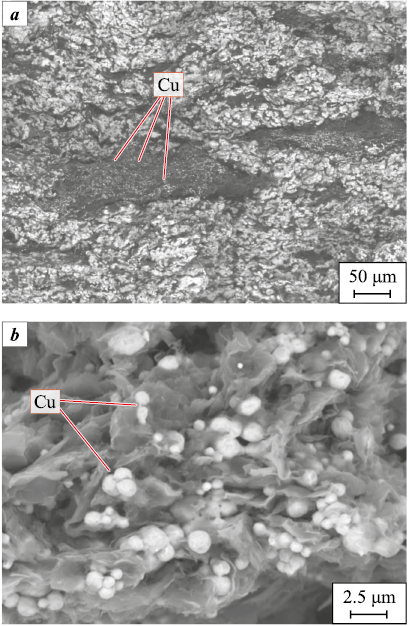
Since the copper acetate solution fails to wet the graphite and does not penetrate deeply, the copper particles in Fig. 5, а appear as clusters and aggregates on the surfaces of graphite particles, with limited presence between the layers (Fig. 5, b). These copper particles on graphite flakes measure about 10–20 µm in size, notably larger than those observed in materials without salts and after treatment with copper sulfate.
Fig. 5. SEM images of a copper-based CM sample |
Samples containing 1 % graphite exhibit lower porosity (П) and consequently, lower electrical resistivity (ρ), while their bending strength (σbend ) surpasses that of CM with 5 % graphite (Table 2). Evidently, achieving high density and strength is challenging due to the elastic nature of graphite and its limited interaction with copper; indeed, some samples with high graphite content experienced destruction during pressing or after sintering.
Table 2. Physical and mechanical properties
|
The treatment of graphite with copper salts significantly influences the structure, as well as the physical and mechanical properties, of the investigated composite materials, a trend clearly observable in samples with 1 % graphite. In materials where graphite remains untreated, nanodispersed copper particles form in low concentrations, resulting in minimal disruption to the graphite crystal structure and yielding the strongest CM samples.
In the structure of graphite treated with copper sulfate, the reduced copper particles are larger and their concentration in the interlayer spaces of graphite flakes is greater higher. Furthermore, after the final sintering, there is a slight increase in porosity, indicating the thermal expansion of the graphite [24; 25]. This may also suggest the completion of copper reduction from sulfate. Consequently, in this material, the bonds in the graphite crystalline lattice are disrupted, defects are formed, and interplanar distances increase. Such structural changes naturally result in increased porosity and decreased strength compared to CM without salts. However, the same material with the highest porosity exhibits lower electrical resistivity than pure copper (ρ = (1.75÷1.80)·10\(^–\)8 mΩм·mm) due to the high concentration of conductive copper particles inside the graphite particles.
After treatment with copper acetate, the amount of reduced copper particles inside the graphite particles is lower than that after sulfate treatment, leading to slightly higher electrical resistivity and strength. However, compared to the material containing untreated graphite the specific resistance of the copper acetate-modified graphite is lower at equal porosity. This is attributed to the presence of copper particles inside the graphite. Additionally, the lower strength value of these samples is a consequence of some disruption of the crystalline structure of the graphite.
Conclusions
The following conclusions were drawn from the experimental research results.
1. An increase in the graphite content from 1 to 5 % in copper-based composites, both with and without salts, leads to higher porosity.
2. Copper acetate undergoes reduction at a temperature of 750 °C during heat treatment in hydrogen, while copper sulfate is reduced at 1000 °C.
3. The copper reduced from copper acetate salt, in the form of large particles, is predominantly observed on the surfaces of graphite flakes.
4. During the sintering of CM, even without graphite treatment with copper salts, copper vaporizes and penetrates inside graphite particles.
5. The samples treated with copper salts, after sintering, exhibit slightly lower strength and reduced electrical resistivity compared to CMs without salts. The decrease in electrical resistivity may be attributed to possible graphite intercalation with copper, while the reduction in strength may be due to emerging defects in the graphite structure.
References
1. Allabergenov B., Kim S. Investigation of electrophysical and mechanical characteristics of porous copper-carbon composite materials prepared by spark plasma sintering. International Journal of Precision Engineering and Manufacturing. 2013;14(7):1177–1183. https://doi.org/10.1007/s12541-013-0160-5
2. Bodnar’ D.M. Metallic and composite thermal conductive materials for powerful semiconductor packages. Komponenty i tehnologii. 2014;12:155–160. (In Russ.).
3. Nanoparticles, nanosystems and their application. Pt. II. Carbon and related layered materials for modern nanoelectronics: Textbook in 2 parts (eds. V.A. Moshnikova, O.A. Alexandrova). Ufa: Ajeterna, 2016. 330 р. (In Russ.).
4. Savich V.V., Oglezneva S.A. Powder metallurgy: Current state and development prospects: monograph. Perm: Publishing house PNIPU, 2021. 695 p. (In Russ.).
5. Emery N., Hérold C., d’Astuto M., Garcia V., Bellin Ch., Marêché J. F., Lagrange P., Loupias G. Superconductivity of Bulk CaC6. Physical Review Letters. 2005.95(8): 087003. https://doi.org/10.1103/PhysRevLett.95.087003
6. Dunaev A.V., Arkhangelsky I.V., Zubavichus Ya.V., Avdeev V.V. Preparation, structure and reduction of graphite intercalation compounds with hexachloroplatinic acid. Carbon. 2008;46(5):788–795. https://doi.org/10.1016/j.carbon.2008.02.003
7. Kyle Kalbus. Copper intercalation into graphite: Theses and Dissertations. Milwaukee (USA), University of Wisconsin Milwaukee, 2012. 34 р. https://dc.uwm.edu/etd/34.
8. Bin X., Сhen Jiazang, Cао Hong, Mа Enbao, Wang Xuehua, Yuan Jizhu. Preparation and structural investigation of CuCl2 graphite intercalation compounds. Acta Geologica Sinica: English Edition. 2008.82(5):1056–1060. https://doi.org/10.1111/j.1755-6724.2008.tb00663.x
9. Mishhenko S.V., Tkachev A.G. Carbon nanomaterials. Production, properties, application: scientific. Moscow: Mashinostroenie, 2008. 320 p. (In Russ.).
10. Kozlov V.V., Kozhitov L.V., Krapukhin V.V., Karpacheva G.P., Pavlov S.A. Highly selective low-temperature nanocomposite Cu/C catalyst for methanol oxidation reaction Izvestiya vysshikh uchebnykh zavedenii. Materialy elektronnoi tekhniki. 2006;3:73–76. (In Russ.).
11. Brandt N.B., Kulbachinsky V.A., Nikitina O.M., Avdeev V.V., Akim V.Ya., Ionov S.G. Supermetallic conductivity and energy spectrum of the third-stage copper chloride intercalation compound in graphite. Pis’ma v ZhTF. 1987;13(5):302–305. (In Russ.).
12. Wang Z., Xu L., Peng J., Tang Z., Han Z., Liu J.. Effect of the microstructure and properties of graphite/copper composites fabricated by microwave sintering. Journal of Materials Science. 2021;56(15):9183–9195. https://doi.org/10.1007/s10853-021-05891-5
13. Oganyan R.A., Zharikov O.V., Oganyan Ya.N., Osip’yan Yu.A. Sintered composite material: Patent 2087575C1 (RF), 1997. (In Russ.).
14. Jia S.Q., Yang F. High thermal conductive copper/diamond composites: State of the art. Journal of Materials Science. 2021;56(3):2241–2274. https://doi.org/10.1007/s10853-020-05443-3
15. Wang J., Ding X., Zhang J., Zhang H., Zhang F., Liu Y., Fan T. Synthesis and properties of surface-modified carbon nanotube/copper composites. Metallurgical and Materials Transactions A. 2019;50(3):1448–1459. https://doi.org/10.1007/s11661-018-05105-9
16. Eroshenko V.D., Ovchinnikov A.N. Improving the tribological and electrical properties of products from a carbon composite material by impregnation with aqueous solutions of copper salts. Izvestija vysshih uchebnyh zavedenij. Severo-Kavkazskij Region. Serija: Tehnicheskie nauki. 2017;2(194):122–126. (In Russ.).
17. Wenhui Zh. Copper-plating graphite composite material and preparation method thereof: Patent CN 101230456 (China). 2008.
18. Yoshihito I., Kenichi O. Copper alloy and method for obtaining a copper alloy: Patent 2510420 (RF). 2010. (In Russ.).
19. Andreeva V.D., Stepanova T.R. Тhe effect of copper atoms on the graphite structure. Technical Physics Letters. 2002;28(9):759–761. https://doi.org/10.1134/1.1511776
20. Oglezneva S.A., Ogleznev N.D., Sirotenko L.D. Study of the relationship between structure and properties of electrodes for EDM tools cutting systems “copper – metal” and “copper – graphite”. Vestnik Uzhno-Uralʹskogo gosudarstvennogo universiteta. Seriya “Mashinostroenie”. 2016;16(1): 63–71. (In Russ.). https://doi.org/10.14529/engin160105
21. Oglezneva S.A., Porozova S.E., Ogleznev N.D., Gilev V.G., Torsunov M.F. Investigation of the interaction in powder materials of the “copper–carbon phases” system for electrode-tools. Metalloobrabotka. 2015;3(87):35–45. (In Russ.).
22. Oglezneva S.A., Porozova S.E., Ogleznev N.D., Kachenyuk M.N. Interaction between copper and thermally expanded graphite during mechanical alloying and spark plasma sintering. Tsvetnye Metally. 2021;(10):86–93. (In Russ.). https://doi.org/10.17580/tsm.2021.10.12
23. Evstifeev E.N., Novikova A.A. Obtaining copper nanoparticles by thermal decomposition of copper formate complex with triethylamine. Mezhdunarodnyi zhurnal prikladnykh i fundamental’nykh issledovaniy. 2017;9:135–139. (In Russ.).
24. Sorokina N.E., Avdeev V.V., Tikhomirov A.S., Lutfullin M.A., Saidaminov M.I. Composite nanomaterials based on intercalated graphite: a textbook. Moscow: MGU, 2010. 50 p. (In Russ.).
25. Belova M.Yu. From black chalk to TRG seals. Armaturostroenie. 2008;1(52):36–43. (In Russ.).
About the Authors
N. D. OgleznevRussian Federation
Nikita D. Ogleznev – Cand. Sci. (Eng.), Engineer at the Department of Mine Survey, Geodesy, and Geoinformation Systems
29 Komsomolsky Prosp., Perm 614990, Russia
I. I. Yakubaev
Russian Federation
Il’ya I. Yakubaev – Graduate Student at the Department of Mechanics of Composite Materials and Structures
29 Komsomolsky Prosp., Perm 614990, Russia
S. А. Oglezneva
Russian Federation
Svetlana A. Oglezneva – Dr. Sci. (Eng.), Professor at the Department of Mechanics of Composite Materials and Structures
29 Komsomolsky Prosp., Perm 614990, Russia
S. Е. Porozova
Russian Federation
Svetlana E. Porozova – Dr. Sci. (Eng.), Professor at the Department of Mechanics of Composite Materials and Structures
29 Komsomolsky Prosp., Perm 614990, Russia
Review
For citations:
Ogleznev N.D., Yakubaev I.I., Oglezneva S.А., Porozova S.Е. Influence of copper salts on the physical and mechanical properties of copper–graphite composite materials. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(2):5-13. https://doi.org/10.17073/1997-308X-2024-2-5-13