Scroll to:
Experimental study of the feasibility of producing materials based on the metastable phase Ti2Fe through explosive compaction and heat treatment
https://doi.org/10.17073/1997-308X-2024-4-17-25
Abstract
The primary regularities in the formation of the structure and phase composition of Fe–Ti system materials, which are promising for hydrogen storage under explosive compaction of titanium and iron powder mixtures, are considered. It has been established that under a loading regime ensuring shock-wave compression pressure P = 11.5 GPa and heating in the falling shock wave to 777 °С, the powder mixture is compacted to an almost non-porous state due to the uniform plastic flow of particles in a direction perpendicular to the direction of shock compression. Under more severe loading conditions (P = 12.5 GPa and t = 831 °С), a monolithic state is also achieved, but the deformation character of the powder mixture component particles changes fundamentally: plastic deformation of the particles is localised in their surface layers and has a pronounced jet character with the formation of specific “vortices”. The influence of the plastic deformation mechanism of powder particles on the formation process of the metastable intermetallic phase Ti2Fe with increased hydrogen capacity has been discovered. It has been established that solid layers of Ti2Fe up to 20 µm thick are formed at the contact boundaries of iron and titanium particles only in the case of jet flows of surface layers of particles. It has been shown that the cause of this effect is the local heating of the contact areas to a temperature above 1085 °С, which according to the phase diagram of the Ti–Fe system, is the minimum temperature for the existence of a liquid phase in it. It has been demonstrated that an effective method for producing materials based on Ti2Fe is the combination of explosive compaction of Fe and Ti powder mixtures and subsequent heat treatment with heating to 1100 °С (reactive sintering in the presence of a liquid phase).
Keywords
For citations:
Krokhalev A.V., Kharlamov V.O., Chernikov D.R., Kuzmin S.V., Lysak V.I. Experimental study of the feasibility of producing materials based on the metastable phase Ti2Fe through explosive compaction and heat treatment. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):17-25. https://doi.org/10.17073/1997-308X-2024-4-17-25
Introduction
The development of hydrogen energy imposes ever-increasing demands on hydrogen storage systems [1]. One of the most promising and safe methods for hydrogen storage is the use of hydride-forming intermetallics [2]. For example, the volumetric density of hydrogen in hydrides based on intermetallics ranges from approximately 60 to 100 kg/m3, whereas in the gaseous state, even at a pressure of 400 bar, it is only about 20 kg/m3 [3]. Such high values of hydrogen capacity enable the creation of anode materials for metal hydride batteries with a discharge capacity reaching 400 mA·h/g [1].
In addition to the currently most widespread intermetallic LaNi5 , one of the most attractive materials for hydrogen storage is the intermetallic TiFe, which is characterized by low cost and the ability to operate at low pressures and ambient temperatures [1].
Currently, many researchers [4–11] are focused on studying the possibility of solving the “activation” problem of this material [12–15]. The issue is that TiFe is very sensitive to air and forms a passivating layer that prevents hydrogen absorption. The classical activation method involves heat treatment, which consists of cyclic exposure to elevated and room temperatures at high hydrogen pressure [16]. However, other methods are now considered more promising, such as mechanical treatment (ball milling, cold rolling, high-pressure torsion) [8; 17–20], the use of increased (compared to stoichiometric) Ti content [5; 9], and the addition of alloying elements such as Mn, Cr, Zr, Y, etc. [6; 10; 21–23].
When using an excess of Ti and alloying, it was found [5; 6; 9; 10; 21–23] that in addition to TiFe, a solid solution based on β-Ti (referred to in some works as the BCC phase or Ti4Fe phase) and the intermetallic Ti2Fe appear in the structure of the material, which act as a kind of “gate” for hydrogen [6–8]. Several studies [4; 5; 9–11] also note a general increase in the hydrogen capacity of materials during primary hydriding, indicating hydrogen absorption not only by the main TiFe phase but also by the secondary β-Ti and Ti2Fe phases. Statistical analysis of data obtained by various authors for three-phase materials based on TiFe, which do not require activation and contain excess amounts of Ti and/or Mn and Zr, showed that their hydrogen capacity during primary hydriding follows the law of additivity and can be described by the equation [24; 25]
H = 0.01537 [TiFe] + 0.03213 [Ti2Fe] + 0.03847 [Ti4Fe],
where H is the hydrogen capacity of the material, wt. %; [TiFe], [Ti2Fe], and [Ti4Fe] – are the contents of the respective phases, wt. %.
As follows from the given equation, the hydrogen capacity of the TiFe phase in the multiphase TiFe–Ti2Fe–Ti4Fe material is about 1.54 wt. % H (which is quite close to the experimental value of 1.70–1.85 wt. % H for the TiFeH hydride given in [10]), the capacity of the BCC solid solution Ti4Fe is 3.85 wt. % H (slightly exceeding the known capacity of 3.7 wt. % H for the Ti4FeH8 hydride [9]), and the capacity of Ti2Fe is about 3.21 wt. % H (also slightly higher than the theoretical estimate of 3.09 wt. % H for the Ti2FeH4.75 hydride given in [9]). It is interesting to note that in the presence of secondary β-Ti and Ti2Fe phases, the main TiFe phase apparently does not form the TiFeH2 hydride with the maximum possible capacity of 1.8–1.98 wt. % H [10], which is more than compensated by the contribution of β-Ti and Ti2Fe.
Another important fact from the statistical analysis [24; 25] and consistent with the conclusions of [9] is that increasing the content of β-Ti in Ti and Fe-based materials reduces their reversible hydrogen capacity, indicating that the saturation of this phase with hydrogen is irreversible. The ability of the intermetallic Ti2Fe to release stored hydrogen depends on the content of alloying elements: in their absence or with a small amount of Mn, hydrogen saturation is reversible [25], but with Zr alloying, ensuring maximum Ti2Fe content in the material’s structure, it is irreversible [9; 25]. This leads to the conclusion that the most promising way to improve Ti–Fe system materials, capable of doubling their reversible hydrogen capacity, is to increase the Ti2Fe content in their structure without using alloying. However, known attempts to solve this problem, based on melting the components followed by annealing [16; 26; 27], have failed [5].
This work explores the possibility of using explosive compaction of iron and titanium powder mixtures for this purpose. The starting point for choosing this method was the well-known fact of frequent intermetallic formation during explosion welding of steels and titanium alloys [28; 29], as well as the fact that during explosive compaction of powders and explosion welding, the area of intense plastic deformation on the surface of the joined components forms similarly [30; 31]. Using powder significantly increases the surface area in the material volume and reduces the effective diffusion paths of elements during interfacial interactions.
Materials and methods
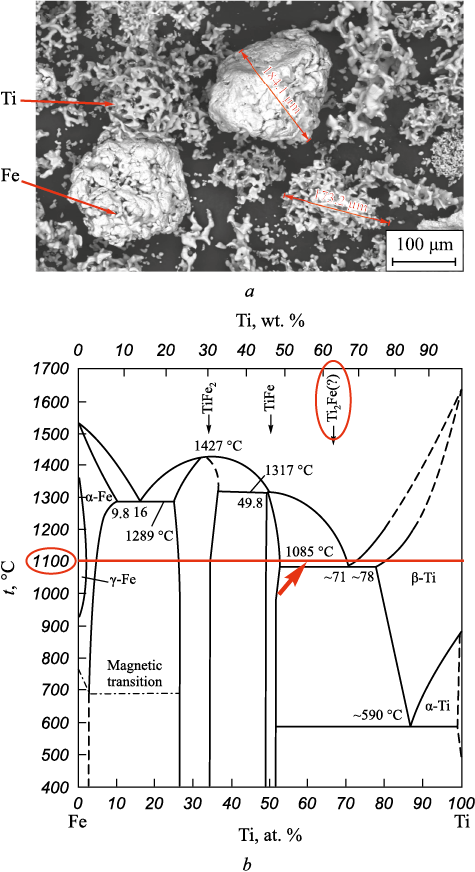
To produce the materials, titanium powder grade PTM-1 and PZhV powder with particle sizes up to 200 µm from a commercial supplier in as-received condition were used. The Ti powder particles had a spongy shape, while the Fe particles were rounded with a pronounced polycrystalline structure (Fig. 1, a). The component content in the powder mixture was 36 wt. % Fe and 64 wt. % Ti, which is nearly stoichiometric for Ti2Fe (see Fig. 1, b) and ensures equal volumetric content of Fe and Ti in the mixture.
Fig. 1. View of Fe and Ti particles in the initial powder mixture (a) |
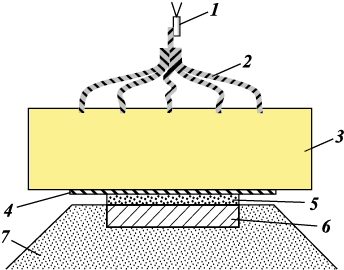
Explosive compaction was performed by placing the initial powder mixture on the surface of a steel substrate and loading it with a flat, normally incident detonation wave through an intermediate layer separating the detonation products from the powder (Fig. 2). The calculation of the physical compression parameters implemented in the experiments was performed using the (P, U)-diagram method [33]. The calculation results are presented in the Table.
Fig. 2. Scheme of explosive loading [33]
Parameters of shock-wave compression of powder mixture
|
The phase composition, structure, and chemical composition of the phases of the obtained samples were investigated by X-ray phase analysis using a D8 Advance X-ray diffractometer (Bruker Optik GmbH, Germany) and scanning electron microscopy using a Versa 3D microscope (FEI, Czech Republic) with an integrated Apollo-X energy-dispersive X-ray microanalysis system (EDAX, USA).
Experimental results
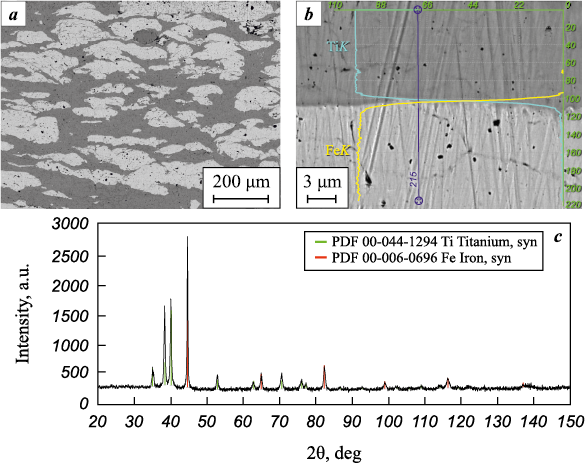
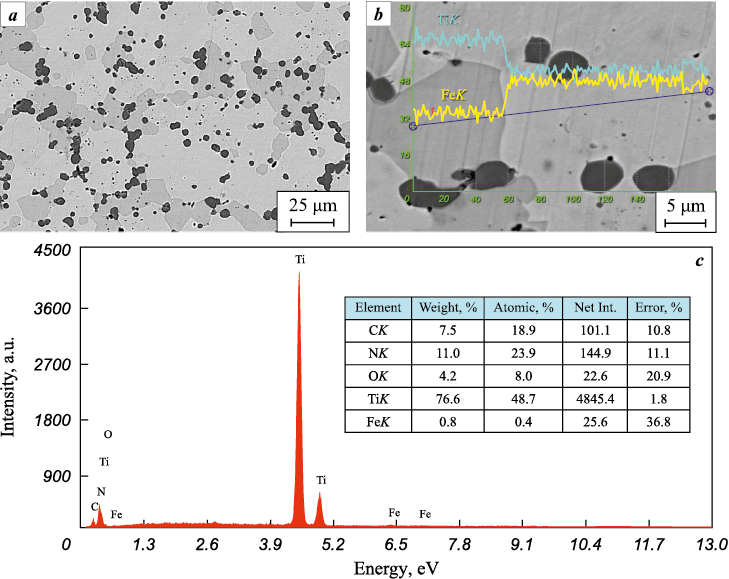
The study of the materials obtained through explosive compaction showed that using this method, which ensures a shock-wave compression pressure of P = 11.5 GPa and a heating temperature up to 777 °C, the powder mixture is compacted into an almost monolithic material (Fig. 3, a). In this process, the deformation of the particles occurs due to uniform plastic flow, resulting in noticeable flattening in the direction of shock compression and spreading in the transverse direction. No traces of changes in the initial phase composition were detected (Fig. 3, c). Even in the immediate vicinity of the interfacial boundaries, the chemical composition of the phases remained practically unchanged (Fig. 3, b).
Fig. 3. Structure (a), chemical composition (b), and phase composition (c) of materials |
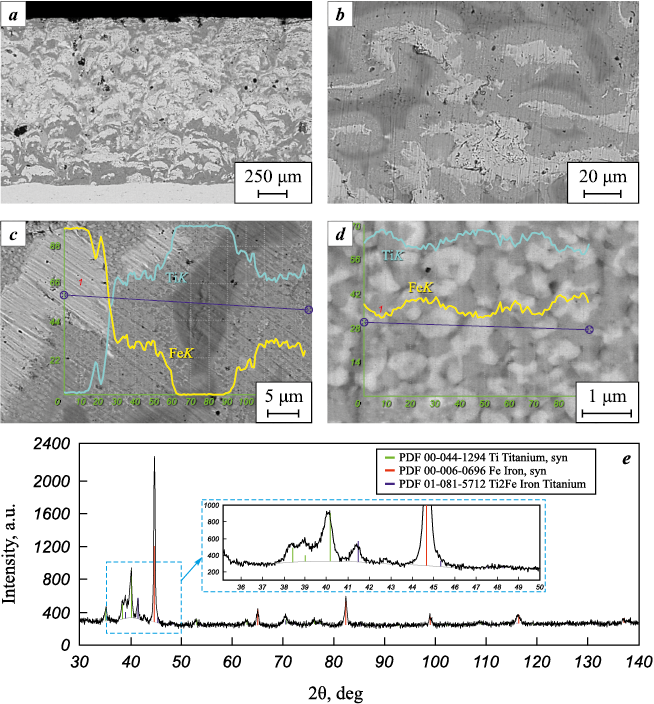
Under more severe shock-wave compression conditions (Р = 12.5 GPa and t = 831 °С) the deformation character of the component particles of the powder mixture changes fundamentally. Plastic deformation localizes in their surface layers and exhibits a pronounced jet character with the formation of specific “vortices,” as described in detail in [33; 35].
At the interfacial surfaces, solid layers up to 20 µm thick of an intermetallic compound (Fig. 4, b) are formed, which, based on its chemical composition (Fig. 4, c) and crystalline structure (Fig. 4, d), can be identified as the metastable phase Ti2Fe.
Fig. 4. Structure (a, b), chemical composition (c, d) and phase (e) composition of materials |
Detailed examination of the microstructure in the interaction zone of the original powder mixture components indicates its chemical heterogeneity, expressed as periodic (with a period of 1.5–2.0 µm) composition oscillations of the intermetallic compound (Fig. 4, d) The deviation from the average stoichiometric ratio of components reaches up to 7 %.
Results and discussion
The data obtained from the conducted studies indicate that the mechanism of plastic deformation of titanium and iron particles during explosive compaction significantly influences the process of forming the metastable phase Ti2Fe. Several hypotheses can be suggested about the nature of this influence. The most plausible hypothesis is that the intermetallic compound forms when a liquid phase is generated in the material during shock-wave treatment. This assertion is primarily supported by the experience of explosive welding of titanium alloys and steels [28; 29; 36].
Given that the contact melting temperature in the Ti–Fe system is low, at 1085 °C according to the phase diagram (see Fig. 1), achieving this temperature at particle boundaries during explosive compaction under conditions ensuring an average calculated heating temperature of 831 °C, combined with jet flows of metal and extreme temperature field heterogeneity, is quite likely. The heterogeneity of the formed intermetallic layer in this case could be a result of the simultaneous growth of its grains from a considerable number of crystallization centers within the liquid phase under rapid cooling due to heat dissipation into the “cold” areas of the structure and the metal substrate.
To test the proposed hypothesis, compacts obtained under loading conditions ensuring uniform plastic flow of the material particles without intermetallic phases were heated to 1100 °C in a vacuum with a holding time of 1 h, followed by cooling in an argon flow. As a result, the initial components of the compacts fully reacted with each other, forming a structure consisting of the intermetallic compounds TiFe and Ti2Fe (Fig. 5, a, b).
Fig. 5. Structure (a), chemical composition of intermetallic phases (b) |
The associated impurity elements – carbon, oxygen, and nitrogen – were bound in titanium oxycarbonitrides (Fig. 5, c). Through coagulation and coalescence in the liquid phase, these formed relatively large inclusions that were uniformly distributed throughout the material (Fig. 5, a, b). The incorporation of part of the Ti into these inclusions appears to be the main reason why the TiFe intermetallic phase formed in addition to the Ti2Fe phase in the material’s structure. Addressing this issue requires increasing the Ti content (above stoichiometric) in the initial powder mixture.
Another factor likely contributing to the presence of the TiFe intermetallic phase in the structure of compacts obtained by heat treatment is the significant increase in the duration of the interfacial interaction process when transitioning from purely explosive treatment to a combination of explosive and heat treatments. As a result, the likelihood of forming the stable TiFe phase and dissolving the metastable Ti2Fe phase increased.
Conclusions
1. Under conditions of explosive compaction of iron and titanium powder mixtures that ensure uniform deformation of the initial particles without jet flows, the powder mixture compacts to an almost non-porous state while maintaining the original phase composition.
2. Using compaction regimes with localized plastic deformation and jet flows of the material particles in the original powder mixture forms a chemically heterogeneous metastable intermetallic Ti2Fe as continuous layers between iron and titanium particles.
3. An effective method for producing materials with a high content of the metastable intermetallic Ti2Fe is to combine explosive compaction of Fe and Ti powder mixtures with subsequent heat treatment in the intercritical temperature range (reactive sintering in the presence of a liquid phase).
References
1. Cuevas F., Amdisen M.B., Baricco M., Buckley C.E., Cho Y.W., De Jongh P., Latroche M. Metallic and complex hydride-based electrochemical storage of energy. Progress in Energy. 2022;4(3):032001. https://doi.org/10.1088/2516-1083/ac665b
2. Tarasov B.P., Fursikov P.V., Volodin A.A., Bocharnikov M.S., Shimkus Y.Y., Kashin A.M., Lototskyy M.V. Metal hydride hydrogen storage and compression systems for energy storage technologies. International Journal of Hydrogen Energy. 2021.46(25):3647–13657. https://doi.org/10.1016/j.ijhydene.2020.07.085
3. Yartys V.A., Lototskyy M.V. Laves type intermetallic compounds as hydrogen storage materials: a review. Journal of Alloys and Compounds. 2022;916:65219.
4. Guéguen A., Latroche M. Influence of the addition of vanadium on the hydrogenation properties of the compounds TiFe0.9Vx and TiFe0.8Mn0.1Vx (x = 0, 0.05 and 0.1). Journal of Alloys and Compounds . 2011; 509(18):5562–5566. https://doi.org/10.1016/j.jallcom.2011.02.036
5. Ulate-Kolitsky E., Tougas B., Huot J. Hydrogenation of TixFe2-x-based alloys with overstoichiometric Ti ratio (x = 1.1, 1.15 and 1.2). International Journal of Hydrogen Energy. 2021;46(77): 38363–38369. https://doi.org/10.1016/j.ijhydene.2021.09.077
6. Manna J., Tougas B., Huot J. First hydrogenation kinetics of Zr and Mn doped TiFe alloy after air exposure and reactivation by mechanical treatment. International Journal of Hydrogen Energy. 2020;45(20):11625–11631. https://doi.org/10.1016/j.ijhydene.2020.02.043
7. Lv P., Liu Z. Effect of high zirconium content on hydrogenation properties and anti-poisoning ability of airexposed TiFe alloy. Journal of Materials Research and Technology. 2019;8(6):5972–5983. https://doi.org/10.1016/j.jmrt.2019.09.072
8. Manna J., Tougas B., Huot J. Mechanical activation of air exposed TiFe + 4 wt% Zr alloy for hydrogenation by cold rolling and ball milling. International Journal of Hydrogen Energy. 2018;43(45):20795–20800. https://doi.org/10.1016/j.ijhydene.2018.09.096
9. Ulate-Kolitsky E., Tougas B., Huot J. First hydrogenation of TiFe with addition of 20 wt.% Ti. Hydrogen. 2022;3(4):379–388. https://doi.org/10.3390/hydrogen3040023
10. Patel A.K., Duguay A., Tougas B., Schade C., Sharma P., Huot J. Microstructure and first hydrogenation properties of TiFe alloy with Zr and Mn as additives. International Journal of Hydrogen Energy. 2020;45(1):787–797. https://doi.org/10.1016/j.ijhydene.2019.10.239
11. Dematteis E.M., Capurso G., Jepsen J., Cuevas F., Latroche M. Fundamental hydrogen storage properties of TiFe-alloy with partial substitution of Fe by Ti and Mn. Journal of Alloys and Compounds. 2021;874:159925. https://doi.org/10.1016/j.jallcom.2021.159925
12. Schober T., Westlake D.G. Activation of FeTi for hydrogen storage: a different view. Scripta Metallurgica. 1981;15(8): 913–918. https://doi.org/10.1016/0036-9748(81)90277-5
13. Silva B.H., Almeida J.M.P., Hernandes A.C., Gonçalves R.V., Zepon G. Pulsed laser activation method for hydrogen storage alloys. International Journal of Hydrogen Energy. 2024;53:885–890. https://doi.org/10.1016/j.ijhydene.2023.12.143
14. Salman M.S., Lai Q., Luo X., Pratthana C., Rambhujun N., Costalin M., Wang T., Sapkota P., Liu W., Grahame A., Tupe J., Aguey-Zinsou K.-F. The power of multifunctional metal hydrides: A key enabler beyond hydrogen storage. Journal of Alloys and Compounds. 2022;920:165936. https://doi.org/10.1016/j.jallcom.2022.165936
15. Lai Q., Sun Y., Wang T., Modi P., Cazorla C., Demirci U.B., Fernandez J.R.A., Leardini F., Aguey-Zinsou K.-F. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Advanced Sustainable Systems. 2019;3(9):1900043. https://doi.org/10.1002/adsu.201900043
16. Reilly J.J., Wiswall R.H. Formation and properties of iron titanium hydride. Inorganic Chemistry. 1974;13(1): 218–222. https://doi.org/10.1021/ic50131a042
17. Lv P., Guzik M.N., Sartori S., Huot J. Effect of ball milling and cryomilling on the microstructure and first hydrogenation properties of TiFe+4 wt.% Zr alloy. Journal of Materials Research and Technology. 2019; 8(2):1828–1834. https://doi.org/10.1016/j.jmrt.2018.12.013
18. Guo F., Namba K., Miyaoka H., Jain A., Ichikawa T. Hydrogen storage behavior of TiFe alloy activated by different methods. Materials Letters: X. 2021;9:100061. https://doi.org/10.1016/j.mlblux.2021.100061
19. Ulate-Kolitsky E., Tougas B., Neumann B., Schade C., Huot J. First hydrogenation of mechanically processed TiFe-based alloy synthesized by gas atomization. International Journal of Hydrogen Energy. 2021; 46(10):7381–7389. https://doi.org/10.1016/j.ijhydene.2020.11.237
20. Oliveira V.B., Beatrice C.A.G., Leal Neto R.M., Silva W.B., Pessan L.A., Botta W.J., Leiva D. R. Hydrogen absorption/desorption behavior of a cold-rolled TiFe intermetallic compound. Materials Research. 2021;24(6):e20210204. https://doi.org/10.1590/1980-5373-MR-2021-0204
21. Yang T., Wang P., Xia C., Liu N., Liang C., Yin F., Li Q. Effect of chromium, manganese and yttrium on microstructure and hydrogen storage properties of TiFe-based alloy. International Journal of Hydrogen Energy. 2020; 45(21):12071–12081. https://doi.org/10.1016/j.ijhydene.2020.02.086
22. Park K.B., Ko W.S., Fadonougbo J.O., Na T.W., Im H.T., Park J.Y., Park H.K. Effect of Fe substitution by Mn and Cr on first hydrogenation kinetics of air-exposed TiFe-based hydrogen storage alloy. Materials Characterization. 2021;178:111246. https://doi.org/10.1016/j.matchar.2021.111246
23. Faisal M., Suh J.Y., Lee Y.S. Understanding first cycle hydrogenation properties of Ti–Fe–Zr ternary alloys. International Journal of Hydrogen Energy. 2021;46(5): 4241–4251. https://doi.org/10.1016/j.ijhydene.2020.11.025
24. Krokhalev A.V., Chernikov D.R., Kharlamov V.O., Tuzhikov O.O., Kuz’min S.V., Lysak V.I. Analysis of the influence of the phase composition of materials of the titanium-iron system on the hydrogen capacity during primary hydrogenation. Izvestija VolgGTU. Ser. Metallurgiya. 2023;7(278):7–13. (In Russ.). https://doi.org/10.35211/1990-5297-2023-7-278-7-13
25. Krokhalev A.V., Chernikov D.R., Kharlamov V.O., Tuzhikov O.O., Kuz’min S.V., Lysak V.I. Analysis of the influence of phase components on the hydrogen capacity of materials in the titanium-iron system. Izvestija VolgGTU. Ser. Problemy materialovedenija, svarki i prochnosti v mashinostroenii. 2023;6(277):47–51. (In Russ.). https://doi.org/10.35211/1990-5297-2023-6-277-47-51
26. Fokin V.N., Fokina E.E., Korobov I.I., Tarasov B.P. Phase transformations in the systems Ti2Fe–H2 and Ti2Fe–NH3. Russian Journal of Inorganic Chemistry. 2016;61(7): 891–895. https://doi.org/10.1134/S0036023616070044.pdf
27. Park K.B., Na T.W., Do Kim Y., Park J.Y., Kang J.W., Kang H.S., Park K., Park H.K. Characterization of microstructure and surface oxide of Ti1.2Fe hydrogen storage alloy. International Journal of Hydrogen Energy. 2021;46(24): 13082–13087. https://doi.org/10.1016/j.ijhydene.2021.01.105
28. Lysak V.I., Kuz’min S.V. Explosion welding. Moscow: Mashinostroenie-1, 2005. 544 p. (In Russ.).
29. Trykov Ju.P., Gurevich L.M., Shmorgun V.G. Titanium-steel composites and compounds: Monograph. Volgograd: VolgGTU, 2013. 344 p. (In Russ.).
30. Bondar’ M.P. Explosive compaction: the type of microstructure of contact boundaries produced by formation of a strong bond. Combustion, Explosion, and Shock Waves. 2004;40(4):489–497. https://doi.org/10.1023/B:CESW.0000033573.06708.d2
31. Bondar M.P. Study of joint formation at the contact of metallic surfaces under dynamic deformation. Physical Mesomechanics. 2001;4(6):61–69. https://cyberleninka.ru/article/n/issledovanie-soedineniy-na-kontaktah-metallicheskih-poverhnostey-sozdannyh-dinamicheskimi-metodami/viewer
32. Phase diagrams of binary metal systems: Handbook. (Ed. N.P. Ljakishev). Moscow: Mashinostroenie, 1997. 1024 p. (In Russ.).
33. Lysak V.I., Krohalev A.V., Kuz’min S.V., Rogozin V.D., Kaunov A.M. Explosive pressing of powders: Monograph. Moscow: Mashinostroenie-1, 2015. 252 p. (In Russ.).
34. Pai V.V., Kuz’min G.E., Yakovlev I.V. Approximate evaluation of loading parameters in composite materials with strong shock waves. Combustion, Explosion, and Shock Waves. 1995;31(3):390–394. https://doi.org/10.1007/BF00742686
35. Rogozin V.D. Explosive processing of powder materials. Volgograd: RPK “Politekhnik”, 2002. 135 p. (In Russ.).
36. Kuz’min E.V., Korolev M.P., Lysak V.I., Kuz’min S.V., Zarubin M.S, Petrushkin P.A., L’vov V.A. Features of the formation of a titanium-steel composite joint during explosion welding under the influence of ultrasound. Izvestija VolgGTU. Ser. Svarka vzryvom i svojstva svarnyh soedinenij. 2020;11(246):19–23. (In Russ.). https://doi.org/10.35211/1990-5297-2020-11-246-19-23
About the Authors
A. V. KrokhalevRussian Federation
Aleksander V. Krokhalev – Dr. Sci. (Eng.). Dean of the Faculty of Structural Materials Technology
28 Lenin Prosp., Volgograd 400005, Russia
V. O. Kharlamov
Russian Federation
Valentin O. Kharlamov – Cand. Sci. (Eng.), Associate Professor at the Department “Equipment and Technology of Welding Production”, Senior Engineer at the Shared Use Center “Physico-chemical research methods“
28 Lenin Prosp., Volgograd 400005, Russia
D. R. Chernikov
Russian Federation
Dmitry R. Chernikov – Postgraduate Student at the Department “Equipment and Technology of Welding Production”
28 Lenin Prosp., Volgograd 400005, Russia
S. V. Kuzmin
Russian Federation
Sergey V. Kuzmin – Dr. Sci. (Eng.), Corresponding Member of the RAS, Professor at the Department “Equipment and Technology of Welding Production”, First Vice-Rector
28 Lenin Prosp., Volgograd 400005, Russia
V. I. Lysak
Russian Federation
Vladimir I. Lysak – Dr. Sci. (Eng.), Academician of the RAS, Professor, Head of the Department “Equipment and Technology of Welding Production”, Scientific Director
28 Lenin Prosp., Volgograd 400005, Russia
Review
For citations:
Krokhalev A.V., Kharlamov V.O., Chernikov D.R., Kuzmin S.V., Lysak V.I. Experimental study of the feasibility of producing materials based on the metastable phase Ti2Fe through explosive compaction and heat treatment. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):17-25. https://doi.org/10.17073/1997-308X-2024-4-17-25
JATS XML