Scroll to:
Dissolution-precipitation and cobalt grain growth during liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials
https://doi.org/10.17073/1997-308X-2024-4-26-34
Abstract
The study presents the results of the dissolution-precipitation process and cobalt grain growth during liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials. The samples were obtained by static pressing of mixtures of technically pure copper, tin, cobalt, and tungsten powders. The average particle size of cobalt was 1.6 µm, and tungsten was 20 µm. Some of the samples contained mechanically activated tungsten with an average particle size of 0.14 µm. Sintering of the materials was carried out in a vacuum at temperatures of 820 and 1100 °C for durations of 5, 20, and 120 min. The structure of the sintered materials was studied using scanning electron microscopy and optical metallography. Elemental distribution maps in the materials were obtained through X-ray microanalysis. The grain sizes of cobalt were measured using specialized software. The largest grain size was observed in the Cu–Sn–Co material: after sintering at the specified temperatures and durations, it ranged from 8 to 46 µm. It was found that the most intensive grain growth occurred within the first 20 min of sintering. The addition of tungsten powder to the Cu–Sn–Co material contributed to the formation of finer cobalt grains. This is explained by the fact that tungsten particles, possessing high surface energy, act as nucleation centers for cobalt crystallization from the liquid phase. Mechanical activation of the tungsten powder increases its free surface area and enhances the mass transfer of Co through the liquid phase to the W particles. This helps to reduce the deposition of material on large Co particles and prevent their growth. As a result, in the Cu–Sn–Co–W material containing mechanically activated tungsten, the minimum average cobalt grain sizes were obtained, ranging from 3 to 25 µm.
Keywords
For citations:
Sokolov E.G., Ozolin A.V., Bobylev E.E., Golius D.A., Arefieva S.A. Dissolution-precipitation and cobalt grain growth during liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):26-34. https://doi.org/10.17073/1997-308X-2024-4-26-34
Introduction
The production of sintered materials with a fine-grained structure and enhanced mechanical properties is a current challenge in powder metallurgy.
During the liquid phase sintering of various materials, a dissolution-precipitation process occurs, which is observed when the solid phase material is soluble in the liquid phase [1–3]. The dissolution-precipitation of the solid phase material contributes to the shrinkage and densification of the material, but it can also lead to the formation of a coarse-grained structure and deteriorate its mechanical properties [4–6].
Various methods are employed to achieve a fine-grained structure in sintered materials, including those aimed at suppressing the dissolution-precipitation process. These methods include homogenizing the sintered mixture by the size and shape of the powder particles [6; 7], reducing the sintering duration [8; 9], and introducing grain growth inhibitors into the powder material [10; 11].
To reduce the duration of the material’s exposure to high temperatures, spark plasma sintering is used [8; 9]. However, this method is not applicable to all materials and products. During spark plasma sintering, the molds wear out rapidly, negatively affecting the dimensional accuracy of the products.
To inhibit grain growth, small amounts of chromium, vanadium, niobium, or other refractory metal carbides are added to tungsten-cobalt hard alloys [10; 11]. There is also evidence of grain growth inhibition in hard alloys with the addition of aluminum oxide nanoparticles [12]. During liquid phase sintering, these substances precipitate on the surface of tungsten carbide particles, inhibiting their growth. However, the addition of these substances leads to the formation of brittle layers around the tungsten carbide particles, which negatively affects the mechanical properties of the sintered materials.
Some composite materials include diamond-graphite nanoparticles, which serve as nucleation centers for the liquid phase crystallization during cooling after sintering [13]. The addition of such particles allows for the formation of fine grains from the liquid phase, but it does not prevent dissolution-precipitation and grain growth of the solid phase during sintering. Thus, there is a need to develop new methods for forming a fine-grained structure in sintered materials.
The study [14] demonstrated that when two dissimilar solid metals, which are only partially soluble in the liquid phase, are placed in a melt, mass transfer occurs towards the metal with higher surface energy. This phenomenon provides opportunities to influence the dissolution-precipitation process by introducing particles with high surface energy into the powder material.
Cu–Sn–Co powder materials are used as metal binders in diamond abrasive tools [15–17], which exhibit resistance to abrasive wear and good adhesion to diamond grains [16]. During the liquid phase sintering of these materials, cobalt grain growth occurs, which negatively impacts their mechanical properties. To prevent this effect, it is advisable to introduce tungsten particles into the Cu–Sn–Co material. Compared to other components of this alloy, tungsten has a higher surface energy, ranging from 2.7 to 5.57 J/m2 according to various sources [18; 19]. Additionally, tungsten is a carbide-forming metal, and its addition improves the adhesion of Cu–Sn–Co binders to diamond abrasive grains [17].
The objectives of this study were as follows:
– to identify the patterns of dissolution-precipitation and cobalt grain growth during the liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials;
– to develop a new method for producing sintered materials with a fine-grained structure.
Research methodology
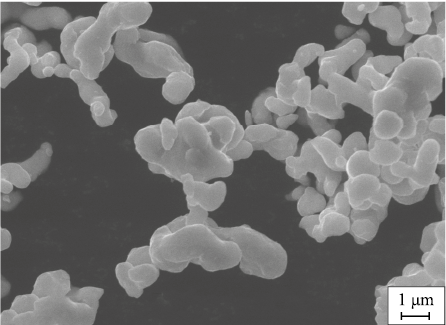
For the research, the following metallic powders were used: PMS-1 copper powder (GOST 4960–75), PO1 tin powder (GOST 9723–73), and Diacob-1600 cobalt powder (Dr. Fritsch Kg., Germany). The latter was obtained by the carbonyl method, with rounded particles averaging 1.6 µm in size (Fig. 1).
Fig. 1. Shape and size of Diacob-1600 cobalt powder particles |
W16.5 special tungsten powder from “Pobedit” JSC (Russia), consisting of equiaxed particles with an average size of about 20 µm, was mechanically activated using a AGO-2U planetary centrifugal mill (NPO “NOVITS”, Russia) for 60 min at a rotational speed of 800 rpm [20]. After treatment, the tungsten particles retained an equiaxed shape and ranged in size from 0.025 to 12.0 µm, with an average size of approximately 0.14 µm.
Mixtures were prepared from the specified powders, and their compositions are given in the Table.
Composition of powder materials
| |||||||||||||||||||||||||||||
Powder samples weighing 20 g were compacted by uniaxial static pressing under a load of 850 MPa. The resulting cylindrical samples, with a diameter of 21 mm, were sintered in a vacuum at temperatures of 820 and 1100 °C for 5, 20 and 120 min, and then microsections were prepared. To reveal the microstructure, an etchant containing 5 g of FeCl3 , 15 ml of HCl, and 100 ml of water was used.
The microstructure of the sintered alloys was studied using a scanning electron microscope EVO HD 15 and a metallographic microscope AxioObserver.A1m (both manufactured by Carl Zeiss AG, Germany) at magnifications of 50–1000×. Grain size measurements in the sintered materials were carried out using AxioVision Rel.4.8 software (Carl Zeiss AG).
The elemental distribution in the samples was investigated by X-ray microanalysis using the EVO HD 15 microscope.
Results and discussion
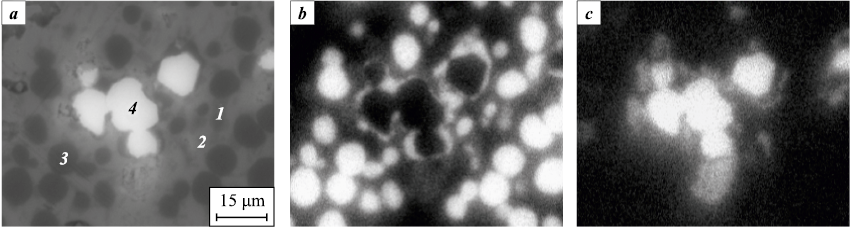
In Fig. 2, the microstructure of the Cu–Sn–Co–W material containing tungsten W16.5, sintered at 820 °C for 20 min, is shown. The phase composition of the Cu–Sn–Co and Cu–Sn–Co–W alloys has been investigated in studies [20; 21].
Fig. 2. Microstructure of the sintered Cu–Sn–Co–W material (а) |
After sintering at temperatures of 820–1100 °C, the materials consist of the following phases: a copper-based solid solution (Cu), the intermetallic phase Cu10Sn3 (ξ-phase), and α-Co. In the Cu–Sn–Co–W samples, α-W and metastable β-W are present. Intermetallic compounds of Co–Sn and Co–W were not detected in the sintered materials. X-ray microanalysis showed that tungsten does not dissolve in the copper-tin phases (Cu and ξ) or in α-Co. The W particles observed in Fig. 2 retained their original shape characteristic of W16.5 powder. In the structure of the material containing mechanically activated tungsten powder, fine W particles remain after sintering, including some with transverse sizes less than 100 nm.
The structural formation of the materials during sintering consisted of several stages:
– formation of the liquid phase: melting of tin, its diffusion into copper particles, and subsequent melting of surface layers enriched with tin;
– viscous flow of the liquid and rearrangement of Co and W particles;
– dissolution-precipitation of cobalt;
– during subsequent cooling, crystallization of the liquid phase into a solid solution (Cu) and intermetallic ξ-phase.
According to X-ray microanalysis, the cobalt content in the (Cu) and ξ phases is 2 wt. % and 3 wt. %, respectively. This indicates its solubility in the liquid phase at sintering temperatures.
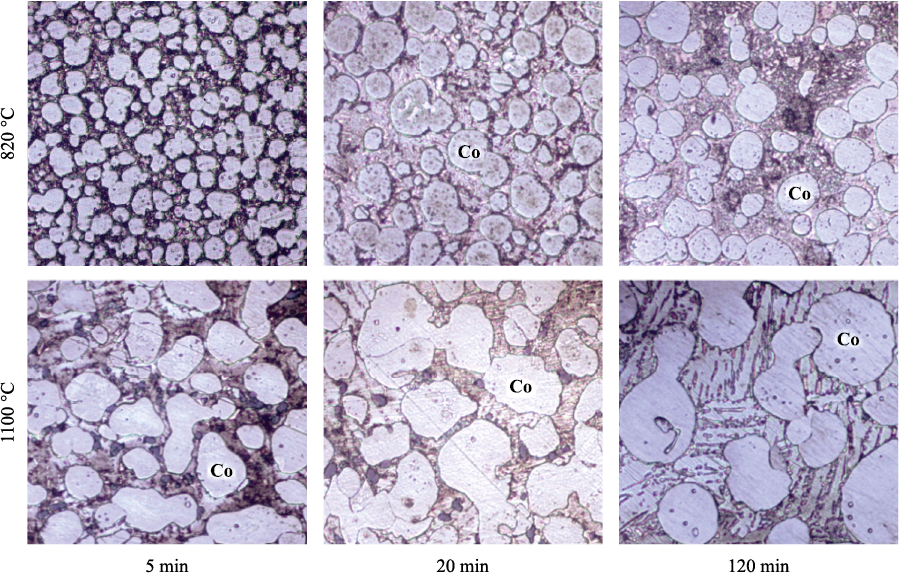
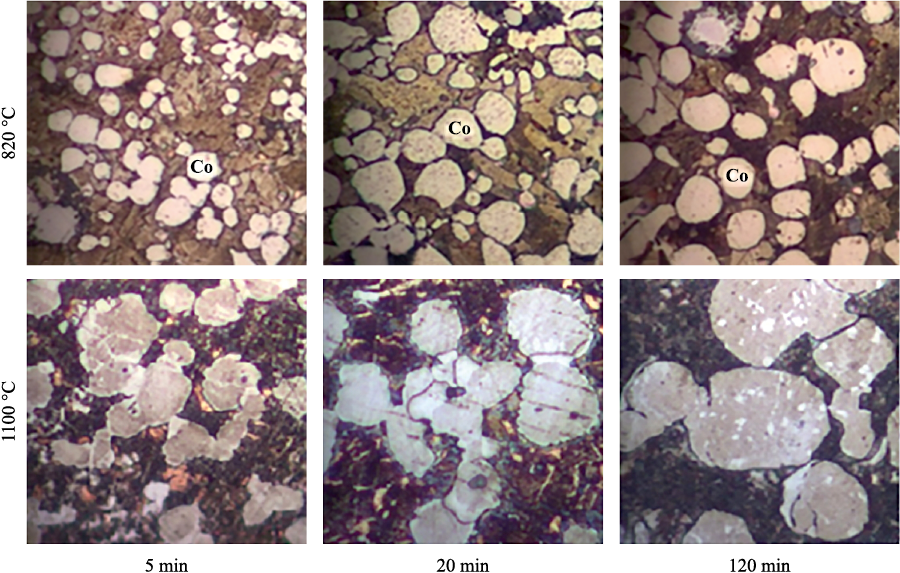
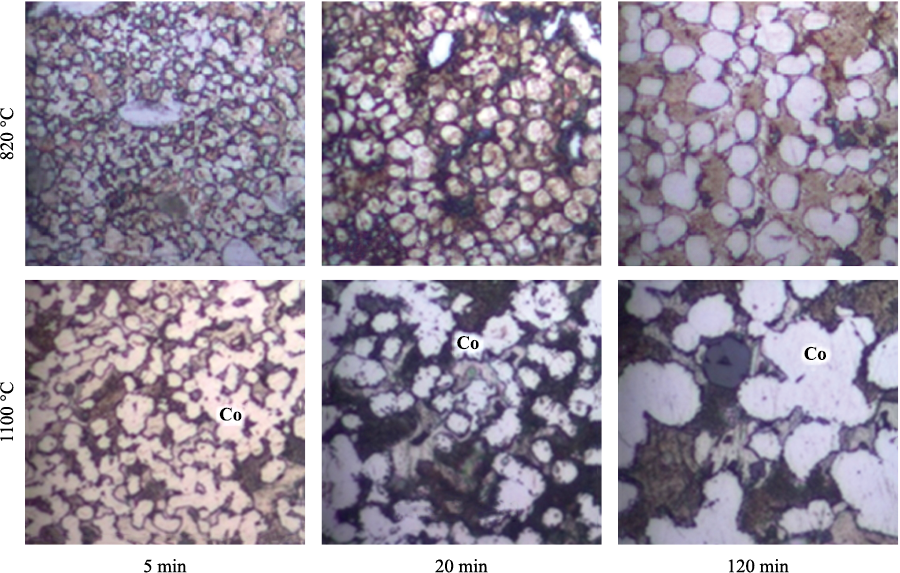
The dissolution-precipitation process leads to noticeable changes in the shape and size of Co particles. In Fig. 3–5, they appear as the brightest component. With increasing temperature and duration of sintering, Co particles become more equiaxed and more uniform in size.
Fig. 3. Structure of Cu–Sn–Co alloys
Рис. 4. Structure of Cu–Sn–Co–W alloys containing tungsten W16.5
Fig. 5. Structure of Cu–Sn–Co–W(m) alloys containing mechanically activated tungsten |
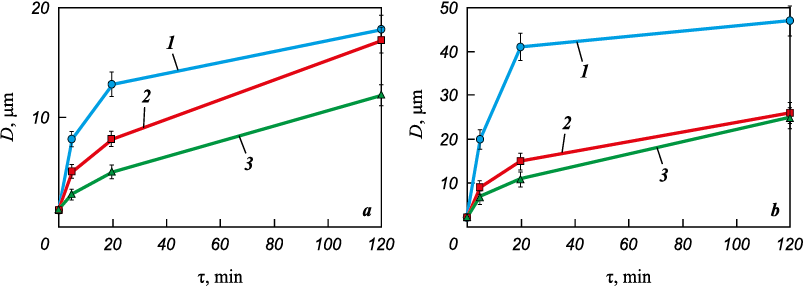
Studies [22; 23] present an equation showing a nonlinear relationship between the duration of liquid phase sintering and the size of the solid phase particle surrounded by the melt:
\[{D^n} - D_0^n = k\tau ,\]
where \({D^n}\) and \(D_0^n\) are the particle sizes after and before sintering; τ is the sintering duration; k is a constant; and the exponent n depends on which stage determines the intensity of the solid phase material’s dissolution-precipitation:
– if the slowest process is the dissolution of the solid phase in the liquid, then n = 2;
– if the determining stage is the diffusion of the dissolved substance in the liquid phase, then n = 3.
The kinetic curves of Co particle growth, shown in Fig. 6, align well with the given equation if the parameter n = 3. This indicates that the rate of Co particle growth is limited by the diffusion rate of Co atoms through the liquid phase.
Fig. 6. Dependence of cobalt grain size on sintering duration at 820 °C (а) and 1100 °C (b) |
In Fig. 6, it is evident that the size of Co particles grows most intensively during the first 5–20 min of sintering. During the dissolution-precipitation process, Co particles become more uniform in shape and size, leading to a decrease in the difference in chemical potentials of cobalt within the particles. As a result, during further isothermal holding, the driving force for mass transfer weakens, and the rate of Co grain growth slows down.
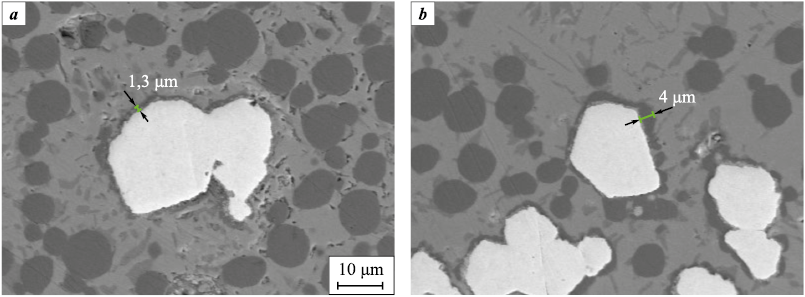
The addition of tungsten to the Cu–Sn–Co powder material significantly reduces the size of Co grains. X-ray microanalysis of the sintered material showed a cobalt layer on the surface of W particles (visible in the component distribution maps, Fig. 2). It is known that tungsten has a higher surface energy than cobalt [18; 19], which facilitates the mass transfer of cobalt through the liquid phase to the surface of W particles during sintering. The thickness of the Co layer is predominantly uniform (Fig. 7, a). Some uneven areas with greater thickness formed due to the sintering of Co particles to W particles (Fig. 7, b). The probable mechanism of sintering is the dissolution of Co at contact points and its precipitation on the free surface of W particles [1; 3].
Fig. 7. Cobalt layer on tungsten particles formed by the precipitation |
It should be noted that, due to their high density, tungsten particles occupy only 2 % of the volume of the sintered material at a mass fraction of 7 %. As a result, the diffusion flux directed from Co particles to W particles is limited by the relatively small free surface area of the tungsten particles.
After mechanical activation under the above conditions, the free surface area of the tungsten powder increases significantly. In the mechanically activated powder, the average particle size of W is about 0.14 µm. Calculations using known geometric formulas show that reducing the diameter of equiaxed (spherical) particles from 20 to 0.14 µm increases the free surface area of the powder by approximately 140 times.
Increasing the free surface area of tungsten enhances the mass transfer of cobalt through the liquid phase to the W particles. As a result, deposition on large Co particles decreases, leading to the formation of a fine-grained structure in the material (see Fig. 5).
The identified patterns of cobalt mass transfer were utilized in developing a new method for producing sintered materials with a fine-grained structure [24]. This method involves introducing fine refractory particles with high surface energy into the powder material. The addition of these particles alters the direction of mass transfer during liquid phase sintering and inhibits the growth of solid phase grains.
The positive effect of introducing mechanically activated tungsten powder into the Cu–Sn–Co material is evident in the following example. At a sintering temperature of 820 °C and a holding time of 20 min, Co grains with an average size of 13 µm form in the material. The introduction of 7 wt. % mechanically activated tungsten powder under the same sintering conditions results in Co grains with an average size of 5 µm.
Conclusions
1. The study determined the patterns of the dissolution-precipitation process of cobalt during the liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials.
2. It was found that the addition of tungsten powder to the Cu–Sn–Co alloy promotes the formation of finer cobalt grains. This is explained by tungsten particles with high surface energy acting as nucleation centers for cobalt crystallization from the liquid phase.
3. Mechanical activation of tungsten powder increases its free surface area and enhances the mass transfer of cobalt through the liquid phase to the W particles. This helps to reduce the deposition of material on large Co particles and further decreases the size of the cobalt grains.
4. Based on the identified patterns, a new method for producing sintered materials with a fine-grained structure was proposed. The essence of this method is that the addition of fine refractory particles with high surface energy to the powder material changes the direction of mass transfer and inhibits the growth of the solid phase grains during liquid phase sintering.
References
1. Kingery W.D. Densification during sintering in the presence of a liquid phase I. Theory. Journal of Applied Physics. 1959;30(3):301–306. https://doi.org/10.1063/1.1735155
2. Eremenko V.N., Naidich Yu.V., Lavrinenko I.A. Sintering in the presence of liquid metal phase. Kiev: Naukova Dumka, 1968. (In Russ.).
3. German R.M., Suri P., Park S.J. Review: Liquid phase sintering. Journal of Materials Science. 2009;44:1–39. https://doi.org/10.1007/s10853-008-3008-0
4. Mortensen A. Kinetics of densification by solution-reprecipitation. Acta Materialia. 1997;45(2):749–758. https://doi.org/10.1016/S1359-6454(96)00202-9
5. Müller D., Konyashin I., Farag S., Ries B., Zaitsev A.A., Loginov P.A. WC coarsening in cemented carbides during sintering. Part I: The influence of WC grain size and grain size distribution. International Journal of Refractory Metals and Hard Materials. 2022;102:105714. https://doi.org/10.1016/j.ijrmhm.2021.105714
6. Trofimenko N.N., Efimochkin I.Yu., Dvoretskov R.M., Batienkov R.V. Production of fine–grained hard alloys of the WC–Co system (Overview). Trudy VIAM. 2020;1(85);92–100. (In Russ.). https://dx.doi.org/10.18577/2307-6046-2020-0-1-92-100
7. Fisher J.G., Kang S-J.L. Strategies and practices for suppressing abnormal grain growth during liquid phase sintering. Journal of the American Ceramic Society. 2019; 102(2):717–735. https://doi.org/10.1111/jace.16008
8. Dudina D.V. Spark plasma sintering of the mixtures of metallic powders and metal matrix composites: Peculiarities of the structure formation and properties of the sintered materials. Obrabotka metallov (tekhnologiya, oborudovanie, instrumenty). 2017;2(75):45–54. (In Russ.). http://dx.doi.org/10.17212/1994-6309-2017-2-45-54
9. Jeong K., Tatami J., Iijima M., Nishimura T. Spark plasma sintering of silicon nitride using nanocomposite particles. Advanced Powder Technology. 2017;28(1):37–42. https://doi.org/10.1016/j.apt.2016.06.027
10. Xie H., Liu Y., Ye J., Li M., Zhu Y., Fan H. Effect of (Cr0.8V0.2)2(C,N) addition on microstructure and mechanical properties of WC–8Co cemented carbides. International Journal of Refractory Metals and Hard Materials. 2014; 47:145–149. https://doi.org/10.1016/j.ijrmhm.2014.07.012
11. Tang J., Liu Y., Ye J.-W., Cao Z.-N., Ma S.-Q., Yang X.-J. Microstructure and mechanical properties improvements in cemented carbides by (Cr,Mo,Ta)2(C,N) inhibitors. Rare Metals. 2021;40(3):679–686. https://doi.org/10.1007/s12598-019-01343-x
12. Su W., Zou J., Sun L. Effects of nano-alumina on mechanical properties and wear resistance of WC–8Co cemented carbide by spark plasma sintering. International Journal of Refractory Metals and Hard Materials. 2020;92:105337. https://doi.org/10.1016/j.ijrmhm.2020.105337
13. Vityaz P.A., Zhornik V.I., Kovalyova S.A., Kukareko V.A. Change of structure and properties of sintered alloys under the influence nanosized carbon additives. Powder Metallurgy аnd Functional Coatings. 2014;(4):12–18. (In Russ.). https://doi.org/10.17073/1997-308X-2014-4-12-18
14. Shatinskii V.F., Zbozhnaya O.M., Maksimovich G.G. Production of diffusion coatings in the medium of low-melting metals. Kiev: Naukova dumka, 1976. 203 p. (In Russ.).
15. Kolodnitskyi V., Bagirov O. On the structure formation of diamond-containing composites used in drilling and stone-working tools (A review). Journal of Superhard Materials. 2017;39:1–17. https://doi.org/10.3103/S1063457617010014
16. Novikov M.V., Mechnyk V.A., Bondarenko M.O., Lyashenko B.A., Kuzin M.O. Composite materials of diamond–(Co–Cu–Sn) system with improved mechanical characteristics. Part 1. The influence of hot re-pressing on the structure and properties of diamond−(Co–Cu–Sn) composite. Journal of Superhard Materials. 2015; 37:402–416. https://doi.org/10.3103/S1063457615060052
17. Sokolov E.G. Ozolin A.V. The influence of temperature on interaction of Sn–Cu–Co–W binders with diamond in sintering the diamond-containing composite materials. Materials Today: Proceedings. 2018;5(12(3)):26038–26041. https://doi.org/10.1016/j.matpr.2018.08.025
18. Vitos L., Ruban A.V., Skriver H.L., Kollár J. The surface energy of metals. Surface Science. 1998;411(1–2):186–202. https://doi.org/10.1016/S0039-6028(98)00363-X
19. Egorov S.N. Calculation of the surface energy of metals in the solid state. Izvestiya vuzov. Severo-Kavkazskii region. Tekhnicheskie nauki. 2003;3:132–134. (In Russ.).
20. Ozolin A., Sokolov E. Effect of mechanical activation of tungsten powder on the structure and properties of the sintered Sn–Cu–Co–W material. Obrabotka metallov (tekhnologiya, oborudovanie, instrumenty). 2022;24(1):48–60. (In Russ.). http://dx.doi.org/10.17212/1994-6309-2022-24.1-48-60
21. Sokolov E.G., Artemev V.P., Voronova M.I. Study of the structure and hardness of alloys of the Sn–Cu–Co system used as diamond abrasive tool binders. Metal Science and Heat Treatment. 2017;59(5-6):321–324. https://doi.org/10.1007/s11041-017-0150-9
22. Ivensen V.A. Phenomenology of sintering and some questions of theory. Moscow: Metallurgiya, 1985. 247 p. (In Russ.).
23. Shlyapin S.D. Supersolidus sintering of powder high-speed steels. Moscow: GINFO; MGIU, 2003. (In Russ.).
24. Sokolov E.G., Ozolin A.V., Golius D.A. Method for obtaining fine-grained structure in sintered materials: Application for invention 2023118770 (RF). 2023. (In Russ.).
About the Authors
E. G. SokolovRussian Federation
Evgeny G. Sokolov – Cand. Sci. (Eng.), Assistant Professor at the Department of Control Systems Engineering, Materials, and Technologies in Mechanical Engineering (CSEMTME)
2 Moskovskaya Str., Krasnodar 350072, Russia
A. V. Ozolin
Russian Federation
Alexander V. Ozolin – Cand. Sci. (Eng.), Junior Research at the Center for Advanced Technologies and Nanomaterials
2 Moskovskaya Str., Krasnodar 350072, Russia
E. E. Bobylev
Russian Federation
Eduard E. Bobylev – Cand. Sci. (Eng.), Assistant Professor at the Department of CSEMTME
2 Moskovskaya Str., Krasnodar 350072, Russia
D. A. Golius
Russian Federation
Dmitry A. Golius – Postgraduate student at the Department of CSEMTME
2 Moskovskaya Str., Krasnodar 350072, Russia
S. A. Arefieva
Russian Federation
Svetlana A. Arefieva – Cand. Sci. (Eng.), Assistant Professor at the Department of CSEMTME
2 Moskovskaya Str., Krasnodar 350072, Russia
Review
For citations:
Sokolov E.G., Ozolin A.V., Bobylev E.E., Golius D.A., Arefieva S.A. Dissolution-precipitation and cobalt grain growth during liquid phase sintering of Cu–Sn–Co and Cu–Sn–Co–W powder materials. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(4):26-34. https://doi.org/10.17073/1997-308X-2024-4-26-34