Scroll to:
Synthesis of fine tungsten powders with low impurity content
https://doi.org/10.17073/1997-308X-2024-5-13-18
Abstract
A chemical-metallurgical method was used to synthesize fine tungsten powders with low oxygen content. The tungsten powders were obtained by hydrogen reduction of tungsten trioxide (WO3 ) powders. Hydrogen was passed through a column with potassium hydroxide for drying. In the first series of experiments, three fractions of WO3 powder of grade “P” 64–100 µm, 40–50 µm, and less than 25 µm were reduced at temperatures of 650, 800, and 950 °C. In the second series of experiments, tungsten powders were obtained by hydrogen reduction of three different WO3 powders of grades “P”, “CP”, and “Tumelom”. The resulting tungsten powders had varying oxygen contents (0.043–2.18 wt. %) and average particle sizes ranging from 35 to 345 nm. X-ray diffraction analysis confirmed the presence of pure tungsten. The minimum oxygen content (0.043 wt. %) in the tungsten powder was achieved by reducing tungsten oxide of grade “CP” at 950 °C for 3 h.
For citations:
Ankudinov A.B., Evstratov E.V., Alymov M.I. Synthesis of fine tungsten powders with low impurity content. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(5):13-18. https://doi.org/10.17073/1997-308X-2024-5-13-18
Introduction
Tungsten powders are widely used in various industries and scientific fields (e.g., radiation shielding for certain medical treatments, nuclear energy, mechanical engineering, etc.) [1–4].
Tungsten is characterized by a high melting point, high thermal conductivity, and low sputtering under plasma exposure, which makes it suitable for the fabrication of internal walls in thermonuclear reactors [5]. However, it is well known that oxygen impurities weaken grain boundaries, thus increasing the risk of cold cracking and leading to a higher ductile-to-brittle transition temperature [6; 7].
Fine tungsten powders are synthesized by various methods. The plasma chemical method is used to produce nanopowders of refractory metals such as W, Mo, Nb, and Ta with average particle sizes ranging from 10 to 100 nm or more [8; 9]. The particles of these powders have a regular shape [10; 11].
Experimental studies on the synthesis of fine tungsten powder from scheelite (CaWO4 ) by the self-propagating high-temperature synthesis (SHS) method were presented in [12; 13]. After leaching the SHS products with a 20 % aqueous hydrochloric acid solution, tungsten powder with a purity of over 99.9 wt. % and particle size less than 0.5 µm was obtained.
In [14], experimental studies on the hydrogen reduction of tungsten acid vapors WO2(OH)2 at around 1000 °C were conducted, resulting in powders containing about 70 wt. % tungsten with a particle size of less than 5 nm.
The work [15] presented experimental studies on the hydrothermal synthesis of porous spherical tungsten oxide particles followed by hydrogen reduction at 600–650 °C. The spherical tungsten particles, measuring tens of microns, consisted of crystallites with sizes ranging from 28 to 37 nm.
In industrial practice, tungsten is typically produced using a chemical-metallurgical method [16; 17], which involves the hydrogen reduction of WO3 . This technology does not require expensive specialized equipment. Tungsten ores are enriched to obtain standard concentrates containing 55–65 wt. % tungsten trioxide (WO3 ). Various technological schemes are used in industrial practice to process concentrates to produce tungsten trioxide, which serves as a precursor for the production of tungsten, tungsten carbide, and other products. The final compounds in the concentrate processing are usually tungsten acid (H2WO4 ) or ammonium paratungstate (APT) 5(NH4 )2O·12WO3·5H2O, which, upon calcination, yield WO3 . Tungsten acid completely loses water at t = 500 °C, while APT decomposes above 250 °C. The calcination temperature of APT depends on the intended use of WO3 .
The reduction process is conducted in tubular furnaces [18] with an excess of dry hydrogen passed over the powder bed (2–4 cm thick) at a rate ensuring the removal of water vapor at temperatures above 630 °C. The primary impurity in tungsten powders is oxygen, the content of which (depending on the reduction mode) ranges from 0.05 to 0.30 wt. % [19; 20].
Advancements in technology demand higher performance characteristics of tungsten powders, including finer particle size and lower oxygen content [21]. Therefore, the practical task of developing a technology for synthesizing fine tungsten powders with low oxygen content is highly relevant. This study aimed to investigate the influence of precursor particle size and reduction temperature on the particle size and oxygen content in tungsten powders.
Materials and methods
Tungsten powder was obtained by hydrogen reduction of three grades of tungsten trioxide (WO3 ) powder: “P” (Pure), “CP” (Chemically Pure) and from LLC “Tumelom”. The hydrogen used conformed to the standard OST 11050.003-83. Hydrogen was passed through a column with potassium hydroxide for drying, ensuring a dew point of approximately –60 °C. The hydrogen flow rate was 1 L/min.
The oxygen content in all powders was analyzed using the infrared absorption method on a “Leco TC-600” (USA) apparatus. The method involves placing a powder sample in a graphite crucible within the analyzer’s furnace, where it melts to form a carbon-saturated melt in a helium atmosphere. The carbon in the molten bath reacts with the sample’s oxygen to form carbon monoxide, which is then flushed out of the furnace by the helium stream. The oxygen content is determined by molecular absorption spectroscopy in the infrared region.
X-ray diffraction analysis was conducted using a “DRON-3M” diffractometer (Burevestnik, Russia) in CuKα radiation (λ = 1.54158 Å). Diffraction patterns were recorded in continuous scanning mode over the 2θ angle range of 20 to 80° with a step size of 0.02°. Phase identification was performed using the “Crystallographica Search Match” software based on the Powder Diffraction File (PDF-2) database.
Specific surface area determination by the BET method was performed according to GOST 2405 on a “TriStar 3000” surface area analyzer (Micromeritics, USA). Scanning electron microscopy (SEM) was carried out on an ultra-high resolution field emission scanning electron microscope “Zeiss Ultra plus” based on “Ultra 55” (Carl Zeiss LLC, Germany).
Results and discussions
First series of experiments. WO3 powder of grade “P” was sieved into three fractions, µm: 64–100, 40–50, and less than 25. These three powders were reduced in a tubular furnace IMETRON for 2 h in a hydrogen stream at temperatures of 650, 800 and 950 °C. A nickel boat containing 5 g of each powder, with a layer thickness of about 3 mm, was placed in a vacuum-tight quartz retort with a diameter of 6 cm and a length of 80 cm. The temperature gradient along the boat was not more than 5 °C. The retort scheme, placed in the furnace, is shown in Fig. 1. Nine reduced tungsten powders were obtained. Table 1 presents the reduction modes for WO3 powder grade “P” and the characteristics of the obtained tungsten powders.
Fig. 1. Scheme of a retort for the reduction of powders
Table 1. Reduction modes for WO3 powder grade “P”
|
Experimental data (Table 1) show that, regardless of the particle size of WO3 , increasing the reduction temperature decreases the oxygen content and increases the average particle size of the tungsten powder.
Second series of experiments. This series was performed on unsieved powders. The reduction of three grades of WO3 powder was conducted for 3 h in a hydrogen stream at 950 °C. Three reduced tungsten powders were obtained (Table 2).
Table 2. Characteristics of tungsten powders obtained
|
When reduced from WO3 grade “CP” at 950 °C for 3 h, the tungsten powder with the lowest oxygen content – 0.043 wt. % – was obtained.
The average particle size (d) of the powders was calculated using the formula d = 6/(ρS), where ρ is the density of tungsten (19.3 g/cm3), and S is the specific surface area of the powder, m2/g.
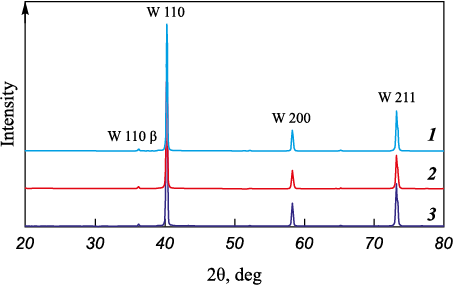
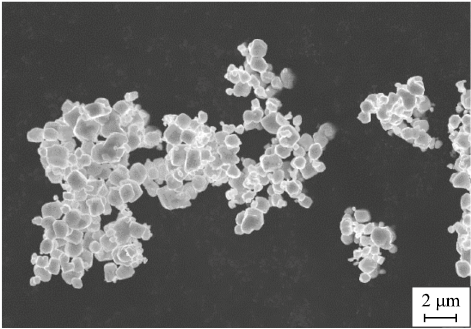
The X-ray diffraction (XRD) results for all powders indicate pure tungsten (Fig. 2). Fig. 3 shows an SEM image of tungsten powder reduced from grade “CP” tungsten oxide at 950 °C for 3 h. The particle shapes of all the powders are similar, regardless of the reduction conditions, with the main difference being particle size.
Fig. 2. X-ray patterns of tungsten powders
Fig. 3. SEM image of tungsten powder reduced from tungsten oxide |
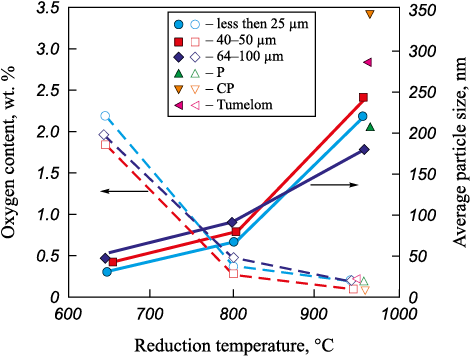
The results of all experiments are shown in Fig. 4. It can be seen that with an increase in the reduction temperature, there is a significant growth in the average particle size of the reduced tungsten powder, regardless of the precursor’s dispersity. Simultaneously, the oxygen content decreases.
Fig. 4. Dependence of oxygen content and average particle size of tungsten powder |
Conclusion
Fine tungsten powders with low oxygen content were synthesized from tungsten trioxide using a chemical-metallurgical method. The minimum oxygen content in the powder was obtained by reducing tungsten oxide of grade “CP” at 950 °C for 3 h. The resulting tungsten powder had the lowest oxygen content of 0.043 wt.% and an average particle size of 345 nm.
References
1. Lassner E., Schubert W.‑D. Tungsten: Properties, chemistry, technology of the element, alloys, and chemical compounds. N.Y.: Springer, 1999, 422 р. https://doi.org/10.1007/978-1-4615-4907-9
2. Povarova K.B. Powder metallurgy of tungsten alloys (Alloying, pretreatment, sintering, TMT, structure, properties). In: Proceedings of 3rd EURO PM 2004 – Powder metallurgy world congress and exhibition (Viena, Austria. 17–21 October 2004). Vol. 5. P. 106–112.
3. Sahin Y. Recent progress in processing of tungsten heavy alloys. Journal of Powder Technology. 2014:764306. https://doi.org/10.1155/2014/764306
4. Chernyak G.B., Povarova K.B. Tungsten in ammunition. Moscow: GNTs RF FGUP “TsNIIKhM”, 2014. 355 p. (In Russ.).
5. Haag J.V., Wang J., Kruska K., Olszta M.J., Henager Jr.C.H., Edwards D.J., Setyawan W., Murayama M. Investigation of interfacial strength in nacre-mimicking tungsten heavy alloys for nuclear fusion applications. Scientific Reports. 2023;13(1):575. https://doi.org/10.1038/s41598-022-26574-4
6. Braun J., Kaserer L., Stajkovic J., Leitz K.-H., Tabernig B., Singer P., Leibenguth P., Gspan C., Kestler H., Leichtfried G. Molybdenum and tungsten manufactured by selective laser melting: Analysis of defect structure and solidification mechanisms. International Journal of Refractory Metals & Hard Materials. 2019;84:104999. https://doi.org/10.1016/j.ijrmhm.2019.104999
7. Veverka J., Vilémová M., Chlup Z., Hadraba H., Lukáč F., Csáki S., Matějíček J., Vontorová J., Chráska T. Evolution of carbon and oxygen concentration in tungsten prepared by field assisted sintering and its effect on ductility. International Journal of Refractory Metals and Hard Materials. 2021;97:105499. https://doi.org/10.1016/j.ijrmhm.2021.105499
8. Krasovskii P.V., Samokhin A.V., Fadeev A.A., Sinayskiy M.A., Sigalaev S.K. Alloying effects and composition inhomogeneity of plasma-created multimetallic nanopowders: a case study of the W–Ni–Fe ternary system. Journal of Alloys and Compounds. 2018;750:265–275. https://doi.org/10.1016/j.jallcom.2018.03.367
9. Samokhin A., Alekseev N., Sinayskiy M., Astashov A., Kirpichev D., Fadeev A., Tsvetkov Y., Kolesnikov A. Nanopowders production and micron-sized powders spheroidization in DC plasma reactors. Powder Technology. 2018;1:1–18. http://doi.org/10.5772/intechopen.76262
10. Krasovskii P.V., Samokhin A.V., Malinovskaya O.S. Characterization of surface oxide films and oxygen distribution in α–W nanopowders produced in a DC plasma reactor from an oxide feedstock. Powder Technology. 2015;286:144–150. http://doi.org/10.1016/j.powtec.2015.07.025
11. Samokhin A.V., Fadeev A.A., Alekseev N.V., Dorofeev A.A., Kalashnikov Ju.P., Sinaisky M.A., Zavertyaev I.D. Processing of tungsten nanopowder into micropowder consisting of spherical particles. Inorganic Materials: Applied Research. 2024;15(2):553–561. https://doi.org/10.1134/S2075113324020369
12. Jung J.C., Ko S.G., Won C.W., Cho S.S., Chun B.S. The self-propagation high-temperature synthesis of ultrafine high purity tungsten powder from scheelite. Journal of Materials Research. 1996;11(7):1825–1830. https://doi.org/10.1557/JMR.1996.0230
13. Ẑivanovič P., Zdujič M., Nikolič Z., Tasič M., Kamberovič Ẑ. Unconventional methods for preparation of fine tungsten powder from scheelite. In: Proceedings of 3rd BMC-2003-Ohnd (R. Macedonia). 2004;10(4):343–353.
14. Ostermann M., Dalbauer V., Schubert W.‑D., Haubner R. Preparation of nano-crystalline tungsten powders from gaseous WO2(OH)2. Tungsten. 2022;4:60–66. https://doi.org/10.1007/s42864-021-00118-1
15. Guo J., Wen X., Wu Y., Xu J., Zhou J. Preparation of spherical tungsten particles assisted by hydrothermal method. Journal of Wuhan University of Technology–Materials Science Edition. 2023;38(6):1457–1462. https://doi.org/10.1007/s11595-023-2842-x
16. Alymov M.I., Ankudinov A.B., Tregubova I.V., Zablotsky A.A. Synthesis of nanopowders on the base of tungsten. Physics and Chemistry of Materials Treatment. 2005;(6):81–82. (In Russ.).
17. Alymov M.I. Powder metallurgy of nanocrystalline materials. Moscow: Nauka, 2007. 169 p. (In Russ.).
18. Alymov M.I., Tregubova I.V., Povarova K.B., Ankudinov A.B., Evstratov E.V. Development of physicochemical foundations for the synthesis of tungsten-based nanopowders with controlled properties. Russian Metallurgy. 2006;(3):217–220. https://doi.org/10.1134/S0036029506030062
19. Zelikman A.N., Nikitina L.S. Tungsten. Moscow: Metallurgiya, 1978. 272 p. (In Russ.).
20. Alymov M.I., Levinskiy Yu.V., Vershinina Е.V., Naboichenko S.S., Kasimtsev A.V., Panov V.S. Metal powders. Products made from metal powders: Directory. Moscow: Infra-Inzheneriya, 2021. 560 p. (In Russ.).
21. Wang R., Zhan X., Chen Y., Zhang C., Che Y., He J. Regulation of particle size and morphology of tungsten powders in bottom-blowing hydrogen reduction process. International Journal of Refractory Metals and Hard Materials. 2024;118:106495. https://doi.org/10.1016/j.ijrmhm.2023.106495
About the Authors
A. B. AnkudinovRussian Federation
Aleksey B. Ankudinov – Senior Research Scientist at the Laboratory of Physical Chemistry of Surfaces and Ultrafine Powder Materials
49 Leninskiy Prosp., Moscow 119334, Russia
E. V. Evstratov
Russian Federation
Evgeniy V. Evstratov – Cand. Sci. (Eng.), Senior Research Scientist at the Laboratory of Physical Chemistry of Surfaces and Ultrafine Powder Materials
49 Leninskiy Prosp., Moscow 119334, Russia
M. I. Alymov
Russian Federation
Mikhail I. Alymov – Dr. Sci. (Eng.), Professor, Corresponding Member of the Russian Academy of Sciences, Head at the Laboratory of Physical Chemistry of Surfaces and Ultrafine Powder Materials
49 Leninskiy Prosp., Moscow 119334, Russia
Review
For citations:
Ankudinov A.B., Evstratov E.V., Alymov M.I. Synthesis of fine tungsten powders with low impurity content. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(5):13-18. https://doi.org/10.17073/1997-308X-2024-5-13-18