Scroll to:
Self-propagating high-temperature synthesis of high-entropy materials: A review
https://doi.org/10.17073/1997-308X-2024-6-5-16
Abstract
High-entropy alloys (HEAs) and compounds, first studied in 2004, represent a new class of materials with promising applications across various technologies and industries. Currently, they include metallic alloys based on disordered solid solutions, ceramic materials based on multicomponent oxides, borides, carbides, silicides, nitrides, and their combinations, as well as ceramic-metal composites. Among the methods for producing high-entropy materials, such as the crystallization of multicomponent melts, mechanical alloying in ball mills, and others, self-propagating high-temperature synthesis (SHS) holds a special place. This review presents the current state of research and development on high-temperature materials produced using the SHS method. It has been shown that the synthesis of metallic high-entropy alloys via SHS is only possible when thermally coupled reactions are employed. This is realized in metallothermic processes and in the synthesis of ceramic-metal composites from elements. The SHS of refractory high-entropy carbides, nitrides, borides, and other compounds can also be performed following the classical element-based synthesis approach. At the same time, the combination of SHS with pre-mechanical alloying of metallic components proves to be effective. For the consolidation of SHS-produced powder products, spark plasma sintering is most commonly used. Additionally, the method of solution combustion synthesis for producing high-entropy ceramics based on oxides is discussed. It has been demonstrated that SHS technology, combined with mechanical activation, mechanical alloying, electric spark plasma sintering, and hot pressing, allows for solving many practical problems in the production of a variety of ceramic, ceramic-metal, and metallic materials based on high-entropy phases.
Keywords
For citations:
Bobozhanov A.R., Rogachev A.S. Self-propagating high-temperature synthesis of high-entropy materials: A review. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):5-16. https://doi.org/10.17073/1997-308X-2024-6-5-16
Introduction
A new class of materials, known as “high-entropy,” encompasses metallic alloys based on disordered solid solutions, ceramic materials based on multicomponent oxides, borides, carbides, silicides, nitrides, and their combinations, as well as ceramic-metal composites. The history of their development spans only two decades – a very short period for metallurgy. The first publications on high-entropy alloys (HEAs) appeared in 2004 [1; 2]. These alloys represented single-phase solid solutions of five or more metals, taken in equal or comparable concentrations. This distinguishes HEAs from traditional alloys, in which the base is typically one metal (at most two), with other components added in small concentrations. Mixing five or more different types of atoms in the crystal structure of disordered solid solutions results in a sufficiently high configurational mixing entropy to stabilize the solid solution.
As is well known from thermodynamics, the stable state of a system corresponds to the minimum value of the Gibbs free energy:
| G = H – TS, | (1) |
where H is enthalpy, S is entropy (considering only the mixing entropy), and T is the absolute temperature in Kelvin. Thus, the stability of a phase at temperature T is ensured if any change in the structure of the phase leads to an increase in Gibbs free energy:
| ΔG = ΔH – TΔS > 0. | (2) |
For example, the decomposition of a disordered solid solution into ordered intermetallic phases (compounds) can be thermodynamically favorable due to a reduction in enthalpy (ΔH < 0), but at the same time, the mixing entropy will decrease as more ordered phases are formed (ΔS < 0), causing the second term in equation (2) to be positive (–TΔS > 0). The phase will remain stable only if the increase in free energy due to the formation of ordered phases is greater than the decrease due to the reduction in enthalpy. The stability condition from (2) can be rewritten as
| \[\left| {\Delta S} \right| > \left| {\frac{{\Delta H}}{T}} \right|.\] | (3) |
Here, the magnitudes of ΔS and ΔH are compared, as both quantities are negative in this case. Therefore, a sufficiently high mixing entropy is required to stabilize the phase. According to Boltzmann’s formula, the mixing entropy of N types of atoms (alloy components) in equal proportions can be approximated as
| ΔS = R lnN, | (4) |
where R is the universal gas constant. The more components there are in an equiatomic alloy, the higher the mixing entropy.
For five or more components
| ΔS ≥ 1.61R = 13.4 J/(mol·K). | (5) |
This is the condition of high entropy that gave these new alloys their name [2; 3]. It should be noted that the stabilizing role of entropy in the formation of multicomponent solid solutions is still not entirely clear. Therefore, in addition to the most common name “high-entropy alloys”, other terms are used in the scientific literature for this class of materials, such as complex concentrated alloys, multi-principal element alloys (MPEAs), and others. The rapid development of HEAs is reflected in thousands of publications, the results of which are analyzed in several reviews [4–11] and monographs [12–14].
Ten to fifteen years after the first publications on HEAs, research on high-entropy ceramics (HECs) began to emerge. Initially, these were multicomponent oxides [15], followed by carbides and nitrides [16–18], borides [19; 20], and other compounds. A common feature of these materials is the presence of five or more types of atoms in the lattice sites of the crystal structure, with smaller non-metal atoms occupying the interstitial spaces between the metallic atoms. Thus, HECs can be considered as solid solutions of several binary compounds. For example, carbides such as TiC, ZrC, HfC, NbC, and TaC can dissolve in each other to form the high-entropy carbide TaNbHfTiZrC5 .
The intense research and development in the field of HEAs and HECs can be explained by their exceptional properties, including high mechanical strength and toughness at both low (down to cryogenic) and high temperatures, wear resistance, heat resistance, and corrosion resistance, as well as unusual electrical and magnetic characteristics. Powder metallurgy methods, particularly mechanical alloying and self-propagating high-temperature synthesis (SHS), are highly promising for the production of HEAs and HECs. To date, there is no specialized review on the application of SHS for the synthesis of HEAs and HECs. Therefore, the goal of our work was to assess the current state of research on SHS of various high-entropy materials – HEAs, HECs, and their composites.
1. SHS of high-entropy metallic alloys
1.1. Thermally coupled reactions
For the SHS process to proceed, sufficient heat must be released during the reaction to sustain the propagation of the combustion wave. This requires a highly negative enthalpy change ΔH. For example, in the reaction Ni + Al = NiAl, the enthalpy is –118 kJ/mol, while in the reaction Ti + C = TiC, it is –209 kJ/mol. Clearly, this contradicts the stability condition (3) for the formation of a disordered solid solution. In [21], the following semi-empirical criterion for HEA formation was proposed:
| \[\Omega = \frac{{{T_m}\Delta S}}{{\Delta H}} \ge 1.1,\] | (6) |
where Tm is the melting temperature of the alloy.
This leads to a constraint on the heat of the SHS reaction:
| G = | ΔH | ≤ 0.91Tm ΔS ≈ 20÷30 kJ/mol. | (7) |
This value is an order of magnitude lower than typical SHS reaction enthalpies. Therefore, direct SHS from elemental metallic HEAs through a reaction such as А + B + C + D + E = ABCDE becomes impossible for two reasons. First, the heat released during the mixing of metals, such as in the Cantor alloy CoCrFeNiMn, is insufficient to sustain a self-propagating reaction. Second, even if five metals are found that release enough heat upon mixing, a solid solution will not form due to the constraint presented in equation (7), and instead, several intermetallic phases will form.
This problem can be solved using the method of thermally coupled reactions proposed by A.G. Merzhanov [22], where two reactions participate in the SHS process – one weakly exothermic and the other strongly exothermic. The heat released from the first reaction provides additional heating for the second. Merzhanov referred to these as “thermally coupled” reactions. This scheme is well-suited for the synthesis of HEAs because the weakly exothermic process of forming a metallic solid solution becomes possible due to the additional heat from another SHS reaction, such as the formation of aluminum oxide, titanium carbide, or similar compounds. Currently, two approaches to thermally coupled synthesis of metallic HEAs can be distinguished, which can be termed the “metallothermic” and “cermet” approaches. Both of these methods are discussed further below.
1.2. Металлотермический синтез ВЭС
The first results on the aluminothermic synthesis of HEAs with the composition CoCrFeNiMnAlx (where x = 0.2÷2.0) were obtained in 2016 [23]. For example, the chemical equation of the coupled reactions for the synthesis of equiatomic HEAs can be written as
0.33Co3O4 + 0.5Cr2O3 + 0.5Fe2O3 + NiO + | (8) |
In this case, the highly exothermic reaction is the oxidation of aluminum, which reduces all the oxides to form molten Al2O3 . The heat released during this process is sufficient to melt all the reduced metals, which then mix to form a six-component metallic melt. The total heat release for this process is 480 kJ per mole of product, and the combustion temperature exceeds 2500 °C, which is higher than the melting points of all the components and products. Since the metallic and oxide melts do not mix and have different specific gravities, they separate under the influence of gravity. Centrifugal machines are used to accelerate this separation by artificially increasing gravitational force by several hundred times. As a result, the metallic melt accumulates at the bottom of the crucible, while the lighter aluminum oxide melt remains at the top. After cooling, two separate crystalline products are formed – a HEA ingot and solid aluminum oxide.
Using this method, HEAs such as СuAlZrTiLix and СuAlZrTi(LiCr)x , which have submicron microstructures and low specific gravities, have also been synthesized [24]. HEAs synthesized by the metallothermic method, like the Cantor alloy CoCrFeNiMn, can be applied in laser welding [25; 26] and friction stir welding [27]. The addition of carbon to the initial thermite powder mixture allowed the production of Cantor alloys strengthened by dispersions of Me23C6-type carbides [28].
HEAs such as CoxCrFeNiAl [29], CoCrFeNiAlxMoy [30] and CoCrFeNiAlxCuy [31] were also synthesized using the metallothermic process, but without the use of a centrifugal separator. The combustion occurred in a powder mixture of oxides and aluminum, with the bulk density placed in a copper crucible. The separation of the metallic melt and slag occurred under natural gravitational forces. This demonstrated the economic efficiency of this method for HEA production.
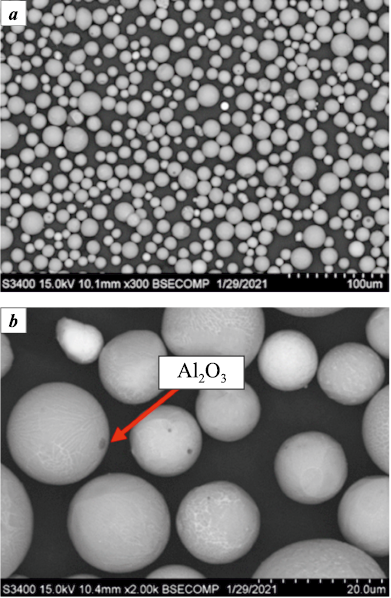
One promising application of metallothermically synthesized HEAs is the production of spherical-shaped powders for additive manufacturing technologies. SHS-produced ingots are crushed and ground in planetary mills, and the resulting narrow powder fractions are then spheroidized in an electric arc plasma torch. Using this method, heat-resistant alloys of NiAl–Cr–Co + 15 %Mo with an average particle size of 14.8 µm were produced [32]. The morphology of the resulting powder is shown in Fig. 1. Individual inclusions of aluminum oxide are observed on the surface of the particles (Fig. 1, b).
Fig. 1. Powder of heat-resistant NiAl–Cr–Co + 15 % Mo alloy |
A four-component (medium-entropy) alloy AlTiVCr was recently obtained via metallothermy without centrifugal forces in steel crucibles [33]. The separation of the metallic melt and slag apparently occurred due to capillary forces, and the solidified alloy and oxide inclusions were then mechanically separated.
Finally, it is worth mentioning the work on the metallothermic synthesis of the AlCoCrFeNi HEA in graphite crucibles within a centrifugal machine [34]. Although the authors of this work claimed the method to be “new,” it essentially replicates the technology described in [23] a year earlier. Moreover, centrifugal SHS-metallothermy has been known for more than 30 years [35], and metallothermy in general for over 120 years [36].
In conclusion, it can be noted that the metallothermic synthesis of HEAs is currently the most developed among the methods for producing metallic HEAs using SHS processes.
1.3. SHS of high-entropy cermets
The production of ceramic-metal composites using the SHS method is also based on the aforementioned principle of thermally coupled SHS reactions. In this case, the highly exothermic reaction is typically synthesis from elements. Reactions between transition metals and carbon or boron are often used, for example:
| Ti + C = TiC + 230 kJ/mol, | (9) |
| Ti + 2B = TiB2 + 290 kJ/mol. | (10) |
As mentioned earlier, the allowable heat for the formation of metallic HEAs is much lower than the heat of these chemical reactions, so the HEA components added to the exothermic mixture act as inert diluents. The SHS reaction scheme can be represented as:
| (1 – x)(Ti + C) + x(Co + Cr + Fe + Ni + Mn) = (1 – x)TiC + xCoCrFeNiMn. | (11) |
The heat released during the reaction between titanium and carbon (9) is sufficient to raise the temperature above the melting points of all the metals. The melts of Co, Cr, Fe, Ni, and Mn combine and, after cooling, crystallize as an HEA. Instead of adding individual metals, pre-prepared HEA powder obtained by other methods (such as mechanical alloying) can be added to the reaction mixture:
| (1 – x)(Ti + C) + xCoCrFeNiMn = (1 – x)TiC + xCoCrFeNiMn. | (12) |
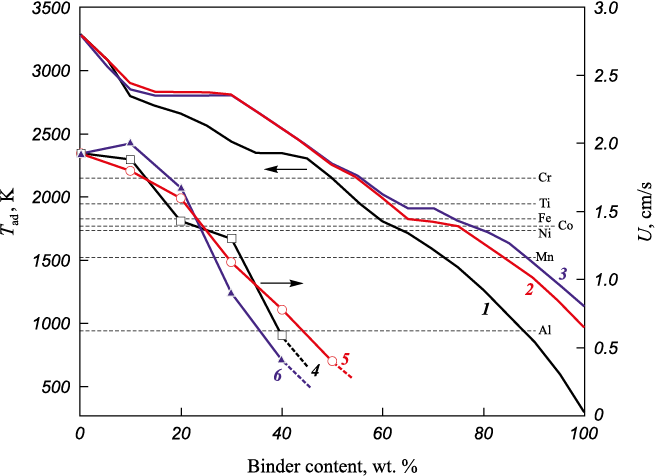
The addition of HEA, either as a mixture of metallic powders or as a pre-synthesized alloy, to the highly exothermic composition leads to a reduction in the combustion temperature. Fig. 2 shows the results of thermodynamic calculations of the adiabatic combustion temperature and experimental measurements of the combustion rate for compositions similar to (12), depending on the HEA content [37]. They indicate that combustion of such mixtures is possible with HEA content up to 40–50 wt. %, provided the combustion temperature remains above the melting points of the metals.
Fig. 2. Adiabatic combustion temperatures (thermodynamic calculation) |
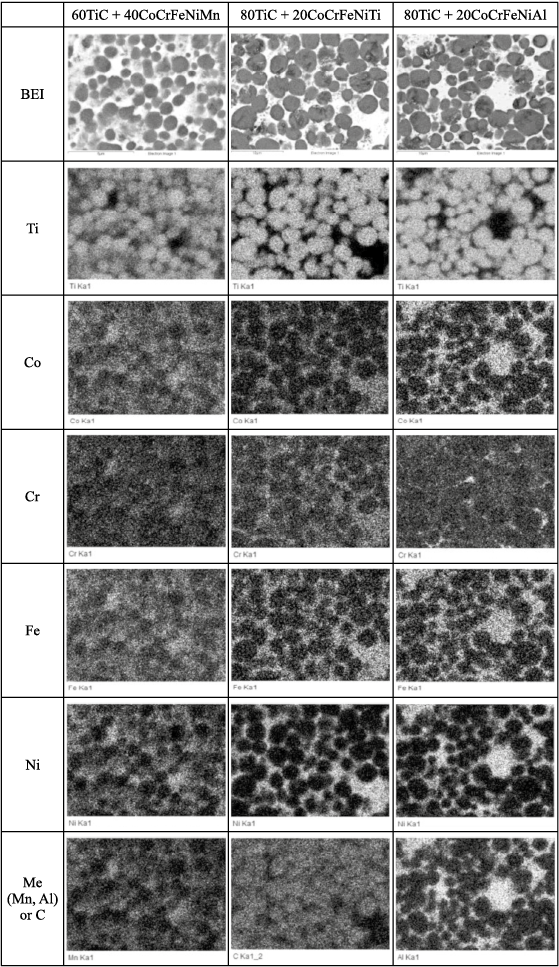
As a result of the SHS reaction, a solid framework of titanium carbide grains is formed, impregnated with a multicomponent metallic melt. Unlike in the metallothermic process, the ceramic and metallic phases do not separate, and upon cooling, a ceramic-metal composite (cermet) forms, consisting of TiC grains and a high-entropy metallic binder. An example of the microstructure and element distribution in such a cermet is shown in Fig. 3. Some features of the structure formation of these materials were studied in [38].
Fig. 3. Microstructure and elemental distribution in some SHS cermets |
To consolidate ceramic-metal composites with a multicomponent binder based on the Cantor alloy, a method of hot SHS pressing was applied, where the workpiece is subjected to quasi-isostatic compression in a sand-filled mold immediately after the combustion wave passes through it [39]. This method, known as the technology for synthetic hard tool materials (STIM technology), had previously been developed for cermets with simpler binder compositions, such as TiC–Ni, TiC–Ni–Mo, TiC–Ni–Cr [35; 36; 40; 41].
The range of ceramic-metallic materials with HEA binders also includes:
• WC–CoCrFeNiMn [42],
• Ti(C,N)–CoCrFeNiAl [43; 44],
• TiB2–CoCrFeNiTiAl [45; 46],
• TiB2–CoCrFeNiAl [47],
• TiB2–TiC–CoCrFeNiTiAl [48] and others.
The authors of [49] proposed considering such materials as a new class of cermets. However, in studies [42–48], pre-synthesized ceramic powders of carbides, borides, and carbonitrides were used, so SHS reactions did not occur. The material was formed during processes such as spark plasma sintering or hot pressing, where heating occurs not due to chemical reactions but from passing an electric current through the mold or the powder itself. There are few studies where such materials are produced without external heating, relying solely on the heat generated from the SHS reaction. However, thermodynamic calculations and initial experimental results indicate that these processes are entirely feasible, suggesting an expansion of research into high-entropy SHS cermets in the near future.
Recently, new types of high-entropy cermets have emerged. In these materials, the high-entropy phase is a multicomponent ceramic, while the binder consists of conventional metals or alloys. These materials will be discussed in the next section.
2. SHS of high-entropy ceramic phases
The crystalline structure of high-entropy ceramic phases consists of two sublattices: a cationic and an anionic one. In the cationic sublattice, there are metal cations of several types (no fewer than five), which are randomly distributed across the lattice sites. This random arrangement provides these compounds with high mixing entropy, which should stabilize the high-entropy ceramics (HECs) and prevent their decomposition into simpler phases. The anionic sublattice consists of atoms of a single type – oxygen, boron, carbon, or nitrogen. Depending on the type of anion, these materials are classified as high-entropy oxides, borides, carbides, or nitrides. It is theoretically possible to combine several anions in the anionic sublattice, such as in carbonitrides or oxycarbonitrides, but the realization of such HECs remains a task for the future. High-entropy ceramics can be considered as solid solutions of corresponding simple compound.
The dissolution of binary compounds into each other under external heating was used to produce the first HECs. Using this method, high-entropy diborides were synthesized [50]:
• (Hf0.2Zr0.2Ta0.2Mo0.2Ti0.2)B2 ,
• (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2 )B2 ,
• (Hf0.2Zr0.2Nb0.2Mo0.2Ti0.2)B2 ,
• (Hf0.2Mo0.2Ta0.2Nb0.2Ti0.2)B2 ,
• (Mo0.2Zr0.2Ta0.2Nb0.2Ti0.2)B2 ,
• (Hf0.2Zr0.2Ta0.2Cr0.2Ti0.2)B2.
In this process, a mixture of simple diborides (e.g., TiB2 , ZrB2 , etc.) was processed for 6 h in a Spex 8000D shaker ball mill (SPEX SamplePrep LLC, USA) and then subjected to spark plasma sintering (SPS) for 5 min at a temperature of 2000 °C and a pressure of 30 MPa. The result was the formation of multicomponent hexagonal phases, some of which have ultra-high melting points. A similar method was used to obtain high-entropy carbides (V0.2Nb0.2Ta0.2Mo0.2W0.2 )C from a mixture of simple carbides, with milling time reduced to 2 h and SPS carried out at 2200 °C and 30 MPa for 10 min [51]. However, this approach is not applicable to SHS. As mentioned earlier, SHS of multicomponent ceramics from mixtures of pre-formed simple compounds is not possible due to insufficient heat from the reaction (with some oxide combinations being an exception). Therefore, to produce HECs via combustion or thermal explosion, mixtures of metallic and non-metallic reagents must be used, which react with significant heat release.
The exothermic SHS reaction can proceed in mixtures of metals with carbon or boron, during mechanical treatment in high-energy planetary ball mills and during SPS. Using this approach, carbides such as (HfTaTiNbZr)C, (HfTaTiNbMo)C and (TiZrHfTaNb)С were synthesized [52–54]. If the chemical reaction leading to the formation of new phases occurs due to heating during SPS, this process is called “reaction SPS” [55]. It can, in principle, be considered a type of SHS process, although there is insufficient data on the temperature conditions inside the molds to determine whether self-heating or the formation of self-propagating combustion waves occurs during mechanical alloying and SPS.
High-entropy carbides such as TaZrHfNbTiС5 [56–59], TaTiNbVWC5 , and TaNbVMoWC5 [58] were directly synthesized using SHS, revealing two key features.
1. Direct synthesis from a mixture of elemental metal and carbon powders often leads to the formation of a multiphase mixture of carbides. This is likely because each metal reacts with carbon separately in the combustion zone. For example, titanium melts at a relatively low temperature (1670 °C) and forms TiC particles before tantalum (3017 °C) melts and reacts with the remaining carbon. Once the simple carbide particles are formed, it becomes difficult to dissolve them into each other. Therefore, a three-stage process was proposed:
– a mixture of metallic powders is processed in planetary mills to form a powder of a high-entropy solid solution of metals;
– carbon (soot) is added to the solution, and additional processing in planetary mills is carried out;
– the resulting reaction mixture is used for SHS of carbides.
2. Some highly refractory HEC components (e.g., Mo, W, V) release little heat during their reaction with carbon, so it is advisable to conduct SHS in the thermal explosion mode, using additional heating of the samples to the point of self-ignition.
Through preliminary mechanical activation of metal mixtures in a planetary mill under an argon atmosphere, followed by SHS in a nitrogen atmosphere, the high-entropy nitride (Hf0.25Ti0.25Cr0.25(FeV)0.25 )N was synthesized in [60], and by combining metallothermic synthesis and SHS from elements, the HEC Al2O3/(NbTaMoW)C was obtained [61].
Finally, a new trend of adding a metallic binder to HECs should be noted [62; 63]. Although such materials, belonging to the class of sintered hard alloys, have so far been produced by traditional powder metallurgy methods, the use of SHS (e.g., via STIM technology) appears to be a promising approach in this area.
3. Synthesis of high-entropy phases
by solution combustion
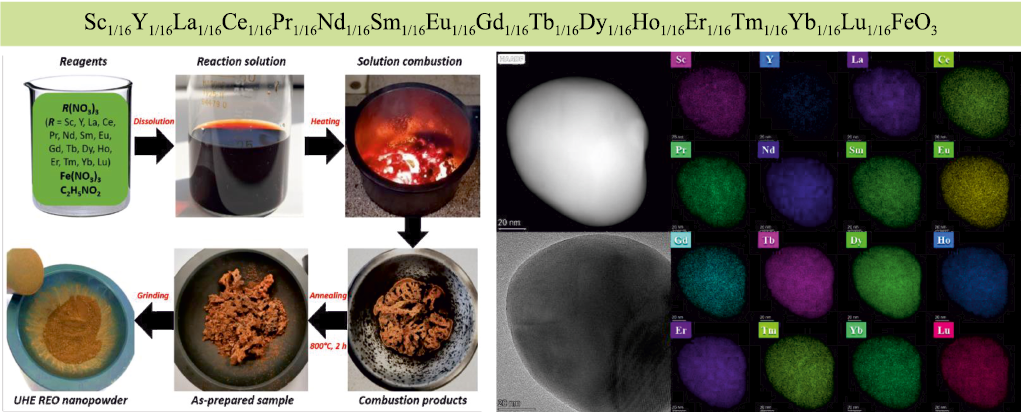
Solution Combustion Synthesis (SCS) is a variation of SHS that allows the production of nanopowders of oxides and other compounds, including multicomponent materials [64]. The process proceeds as follows: metal nitrates are dissolved in water along with an organic compound (such as glycine, urea, or citric acid). The solution is then heated to a relatively low temperature of 120–140 ºC, causing the water to evaporate, after which the resulting gel ignites. The combustion product is a loose powder composed of particles ranging in size from 10 to 100 nm. Since all the components are mixed at the molecular level in the solution, they are also uniformly distributed in the nanoparticles of the product. This enables the combination of many elements in solid solutions and single-phase compounds. Using this method, high-entropy oxides such as (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O have been produced. Another example is a “record-breaking” ferrite in terms of the number of components [66], which exhibits valuable magnetic properties. The schematic of the SCS process and X-ray maps showing the homogeneous distribution of many elements in the nanoparticles of the product are presented in Fig. 4.
Fig. 4. Scheme of the solution combustion synthesis process and distribution |
Conclusion
The SHS technology, in combination with mechanical activation, mechanical alloying, spark plasma sintering, and hot pressing, provides solutions to many practical challenges in the production of various ceramic, ceramic-metal, and metallic materials based on high-entropy phases. Possible technological pathways for producing such materials are illustrated in the diagram in Fig. 5. It seems highly likely that this scientific and technological field will experience rapid development in the coming years.
Fig. 5. Scheme of possible pathways for obtaining high-entropy materials using SHS |
References
1. Cantor B., Chang I.T.H., Knight P., Vincent A.J.B. Microstructural development in equiatomic multicomponent alloys. Materials Science and Engineering: A. 2004;375-377: 213–218. https://doi.org/10.1016/j.msea.2003.10.257
2. Yeh J.-W., Chen S.-K., Lin S.J., Gan J.-Y., Chin T.-S., Shun T.-T., Tsau C.-H., Chang S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel design concepts and outcomes. Advanced Engineering Materials. 2004;6(5):299–303. https://doi.org/10.1002/adem.200300567
3. Yeh J.-W. Recent progress in high-entropy alloys. Annales De Chimie – Science des Materiaux. 2006;31(6):633–648. https://doi.org/10.3166/ACSM.31.633-648
4. Zhang Y., Zuo T.T., Tang Z., Gao M.C., Dahmen K.A., Liaw P.K., Lu Z.P. Microstructures and properties of high-entropy alloys. Progress in Materials Science. 2014;61: 1–93. https://doi.org/10.1016/j.pmatsci.2013.10.001
5. Cantor B. Multicomponent and high entropy alloys. Entropy. 2014;16(9):4749–4768. https://doi.org/10.3390/e16094749
6. Alaneme K.K., Bodunrin M.O., Oke S.R. Processing, alloy composition and phase transition effect on the mechanical and corrosion properties of high entropy alloys: a review. Journal of Materials Research and Technology. 2016;5(4):384–393. http://doi.org/10.1016/j.jmrt.2016.03.004
7. Miracle D.B., Senkov O.N. A critical review of high entropy alloys and related concepts. Acta Materialia. 2017;122: 448–511. https://doi.org/10.1016/j.actamat.2016.08.081
8. Zhang W., Liaw P.K., Zhang Y. Science and technology in high-entropy alloys. Science China Materials. 2018;61(1): 2–22. https://doi.org/10.1007/s40843-017-9195-8
9. Rogachev A.S. Structure, stability, and properties of high-entropy alloys. Physics Metals and Metallography. 2020;121(8):733–764. https://doi.org/10.1134/S0031918X20080098
10. Cantor B. Multicomponent high-entropy Cantor alloys. Progress in Materials Science. 2021;120:1–36. https://doi.org/10.1016/j.pmatsci.2020.100754
11. Bridges D., Fieser D., Santiago J.J., Hu A. Novel frontiers in high-entropy alloys. Metals. 2023;13(7):1193. https://doi.org/10.3390/met13071193
12. Murty B.S., Yeh J.W., Ranganathan S., Bhattacharjee P.P. High-entropy alloys. 2nd ed. Amsterdam: Elsevier, 2019. 374 p. https://doi.org/10.1016/C2017-0-03317-7
13. Zhang Y. High-entropy materials. A brief introduction. Singapore: Springer Nature, 2019. 159 p. https://doi.org/10.1007/978-981-13-8526-1
14. Jamieson B., Liaw P.K. (Ed.). High-entropy materials: Theory, experiments, and applications. Switzerland AG: Springer Nature, 2021, 774 p. https://doi.org/10.1007/978-3-030-77641-1
15. Rost C.M., Sachet E., Borman T., Moballegh A., Dickey E.C., Hou D., Jones J.L., Curtarolo S., Jon-Paul M. Entropy-stabilized oxides. Nature Communications. 2015; 6(1):8485. https://doi.org/10.1038/ncomms9485
16. Dusza J., Švec P., Girman V., Sedlák R., Castle E.G., Csanádi T., Kovalčíková A., Reece M.J. Microstructure of (Hf–Ta–Zr–Nb)C high-entropy carbide at micro and nano/atomic level. Journal of the European Ceramic Society. 2018; 38(12):4303–4307. https://doi.org/10.1016/j.jeurceramsoc.2018.05.006
17. Castle E., Csanádi T., Grasso S., Dusza J., Reece M. Processing and properties of high-entropy ultra-high temperature carbides. Scientific Reports. 2018;8609(8):1–12. https://doi.org/10.1038/s41598-018-26827-1
18. Yang Y., Ma L., Gan G.-Y., Wang W., Tang B.-Y. Investigation of thermodynamic properties of high entropy (TaNbHfTiZr)C and (TaNbHfTiZr)N. Journal of Alloys and Compounds. 2019;788:1076–1083. https://doi.org/10.1016/j.jallcom.2022.164526
19. Gu J., Zou J., Sun S.-K., Wang H., Yu S.-Y., Zhang J., Wang W., Fu Z. Dense and pure high-entropy metal diboride ceramics sintered from self-synthesized powders via boro/carbothermal reduction approach. Science China Materials. 2019; 62(12):1898–1909. https://doi.org/10.1007/s40843-019-9469-4
20. Tallarita G., Licheri R., Garroni S., Orrù R., Cao G. Novel processing route for the fabrication of bulk high-entropy metal diborides. Scripta Materialia. 2019;158:100–104. https://doi.org/10.1016/j.scriptamat.2018.08.039
21. Yang X., Zhang Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Materials Chemistry and Physics. 2012;132:233– 238. https://doi.org/10.1016/j.matchemphys.2011.11.021
22. Merzhanov A.G. Thermally coupled processes of self-propagating high-temperature synthesis. Doklady Academii nauk. 2010;433(5):1–4. (In Russ.).
23. Sanin V.N., Yukhvid V.I., Ikornikov D.M., Andreev D.E., Sachkova N.V. SHS metallurgy of high-entropy transition metal alloys. Doklady Physical Chemistry 2016;470: 145–149. https://doi.org/10.1134/S001250161610002X
24. Sanin V.N., Ikornikov D.M., Andreev D.E., Sachkova N.V., Yukhvid V.I. Synthesis of cast high entropy alloys with a low specific gravity by centrifugal metallothermic SHS-methods. Advanced Materials & Technologies. 2017;(3):24–33. https://doi.org/10.17277/amt.2017.03.pp.024-033
25. Kashaev N., Ventzke V., Stepanov N., Shaysultanov D., Sanin V., Zherebtsov S. Laser beam welding of a CoCrFeNiMn-type high entropy alloy produced by self-propagating high-temperature synthesis. Intermetallics. 2018;96:63–71. https://doi.org/10.1016/j.intermet.2018.02.014
26. Kashaev N., Ventzke V., Petrov N., Horstmann M., Zherebtsov S., Shaysultanov D., Sanin V., Stepanov N. Fatigue behaviour of a laser beam welded CoCrFeNiMn-type high entropy alloy. Materials Science and Engineering: A. 2019;766:138358. https://doi.org/10.1016/j.msea.2019.138358
27. Shaysultanov D., Stepanov N., Malopheyev S., Vysotskiy I.V., Sanin V.V., Mironov S., Kaibyshev R., Salishchev G., Zherebtsov S. Friction stir welding of a сarbon-doped CoCrFeNiMn high-entropy alloy. Materials Characterization. 2018;145:353–361. https://doi.org/10.1016/j.matchar.2018.08.063
28. Klimova M., Shaysultanov D., Chernichenko R.S., Sanin V.N., Stepanov N., Zherebtsov S., Belyakov A.N. Recrystallized microstructures and mechanical properties of a C-containing CoCrFeNiMn-type high-entropy alloy. Materials Science and Engineering: A. 2019;740:201–210. https://doi.org/10.1016/j.msea.2018.09.113
29. Kaya F., Yetiş M., Selimoğlu G. İ., Derin B. Influence of Co content on microstructure and hardness of AlCoxCrFeNi (0 ≤ x ≤ 1) high-entropy alloys produced by self-propagating high-temperature synthesis. Engineering Science and Technology, an International Journal. 2022;27:101003. https://doi.org/10.1016/j.jestch.2021.05.007
30. Kaya F., Dizdar K.C., Aliakbarlu S., Selimoğlu G.İ., Derin B. Self-propagating high-temperature synthesis of AlxCoCrFeNiMoy high-entropy alloys: Thermochemical modelling, microstructural evaluation and high temperature oxidation behaviour. Materials Chemistry and Physics. 2024;318:129304. https://doi.org/10.1016/j.matchemphys.2024.129304
31. Kaya F., Erşan A.A., Çayır E., Kirim T., Duygulu Ö., Selimoğlu G.İ., Derin B. Cost-effective synthesis and thermomechanical processing of AlxCoCrFeNiCuy (x&y = 0.5, 1) high-entropy alloys. Materials Chemistry and Physics. 2024;311:128554. https://doi.org/10.1016/j.matchemphys.2023.128554
32. Sanin V.V., Kaplansky Yu.Yu., Aheiev M.I., Levashov E.A., Petrzhik M.I., Bychkova M.Ya., Samokhin A.V., Fadeev A.A., Sanin V.N., Heat-resistant alloys NiAl–Cr–Co–X (X = La, Mo, Zr, Ta, Re) and fabrication of powders for additive manufacturing. Materials. 2021;14:3144. https://doi.org/10.3390/ma14123144
33. Dastjerdi Z., Sharifitabar M., Afarani M. S. Preparation of a hard AlTiVCr compositionally complex alloy by self-propagating high-temperature synthesis. International Journal of Refractory Metals and Hard Materials. 2024;122:106694. https://doi.org/10.1016/j.ijrmhm.2024.106694
34. Li R.X., Liaw P.K., Zhang Y., Synthesis of AlxCoCrFeNi high-entropy alloys by high-gravity combustion from oxides. Materials Science and Engineering: A. 2017; 707:668–673. https://doi.org/10.1016/j.msea.2017.09.101
35. Borovinskaya I.P., Gromov A.A., Levashov E.A., Maksimov Yu.M., Mukasyan A.S., Rogachev A.S. (eds.). Concise encyclopedia of SHS. Elsevier, 2017. 438 p. https://doi.org/10.1016/C2015-0-00439-7
36. Rogachev A.S., Mukasyan A.S. Combustion for material synthesis. 1st ed. CRC Press Taylor & Francis Group, 2014. 424 p. https://doi.org/10.1201/b17842
37. Rogachev A.S., Vadchenko S.G., Kochetov N.A., Kovalev D.Yu., Kovalev I.D., Shchukin A.S., Gryadunov A.N., Baras F., Politano O. Combustion synthesis of TiC-based ceramic-metal composites with high entropy alloy binder. Journal of the European Ceramic Society. 2020;40(7):2527–2532. https://doi.org/10.1016/j.jeurceramicsos.2019.11.059
38. Vadchenko S.G., Vergunova Yu.S., Rogachev A.S., Kovalev I.D., Mukhina N.I. Formation of products upon ignition, combustion and melting of mixtures of high-entropy alloy FeNiCoCrCu with titanium and carbon. Powder Metallurgy аnd Functional Coatings. 2023;17(1):28–38. https://doi.org/10.17073/1997-308X-2023-1-28-38
39. Rogachev A.S., Gryadunov A.N., Kochetov N.A., Shchukin A.S., Baras F., Politano O. High-entropy-alloy binder for TiC-based cemented carbide by SHS method. International Journal of Self-Propagating High-Temperature Synthesis. 2019;28:196–198. https://doi.org/10.3103/S1061386219030117
40. Merzhanov A.G. Self-propagating high-temperature synthesis: Twenty years of research and findings. In: Combustion and plasma synthesis of high-temperature materials. Munir Z., Holt J.B. (Eds.). New York: VCH, 1990. P. 1–53.
41. Levashov E.A., Mukasyan A.S., Rogachev A.S., Shtansky D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. International Materials Reviews. 2017;62(4):203–239. https://doi.org/10.1080/09506608.2016.1243291
42. Velo I.L., Gotor F.J., Alcala M.D., Real C., Cordoba J.M. Fabrication and characterization of WC-HEA cemented carbide based on the CoCrFeNiMn high entropy alloy. Journal of Alloys and Compounds. 2018;746:1–8. https://doi.org/10.1016/j.jallcom.2018.02.292
43. Zhu G., Liu Y., Ye J. Fabrication and properties of Ti(C,N)-based cermets with multi-component AlCoCrFeNi high-entropy alloys binder. Materials Letters. 2013;113:80–82. http://dx.doi.org/10.1016/j.matlet.2013.08.087
44. Zhu G., Liu Y., Ye J. Early high-temperature oxidation behavior of Ti(C,N)-based cermets with multi-component AlCoCrFeNi high-entropy alloy binder. International Journal of Refractory Metals and Hard Materials. 2014;44:35–41. http://dx.doi.org/10.1016/j.ijrmhm.2014.01.005
45. Ji W., Zhang J., Wang W., Wang H., Zhang F., Wang Y., Fu Z. Fabrication and properties of TiB2-based cermets by spark plasma sintering with CoCrFeNiTiAl high-entropy alloy as sintering aid. Journal of the European Ceramic Society. 2015;35(3):879–886. http://dx.doi.org/10.1016/j.jeurceramsoc.2014.10.024
46. Fu Z., Koc R. Ultrafine TiB2–TiNiFeCrCoAl high-entropy alloy composite with enhanced mechanical properties. Materials Science and Engineering: A. 2017;702:184–188. http://dx.doi.org/10.1016/j.msea.2017.07.008
47. Zhang S., Sun Y., Ke B., Li Y., Ji W., Wang W., Fu Z., Preparation and characterization of TiB2-(supra-nano-dual-phase) high-entropy alloy cermet by spark plasma sintering. Metals. 2018;58(8):1–10. https://doi.org/10.3390/met8010058
48. Fu Z., Koc R. TiNiFeCrCoAl high-entropy alloys as novel metallic binders for TiB2–TiC based composites. Materials Science and Engineering A. 2018;735:302–309. https://doi.org/10.1016/j.msea.2018.08.058
49. De la Obra A.G., Avilés M.A., Torres Y., Chicardi E., Gotor F.J. A new family of cermets: Chemically complex but microstructurally simple. International Journal of Refractory Metals and Hard Materials. 2017;63:17–25. http://dx.doi.org/10.1016/j.ijrmhm.2016.04.011
50. Gild J., Zhang Y., Harrington T., Jiang S., Hu T., Quinn M.C., Mellor W.M., Zhou N., Vecchio K., Luo J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultrahigh temperature ceramics. Scientific Reports. 2016;6(1):37946. https://doi.org/10.1038/srep37946
51. Harrington T.J., Gild J., Sarker P., Toher C., Rost C.M., Dippo O.F., McElfresh C., Kaufmann K., Marin E., Borowski L., Hopkins P.E., Luo J., Curtarolo S., Brenner D.W., Vecchio K.S. Phase stability and mechanical properties of novel high entropy transition metal carbides. Acta Materialia. 2019;166:271–280. https://doi.org/10.1016/j.actamat.2018.12.054
52. Moskovskikh D.O., Vorotilo S., Sedegov A.S., Kuskov K.V., Bardasova K.V., Kiryukhantsev-Korneev P.V., Zhukovskyi M., Mukasyan A.S. High-entropy (HfTaTiNbZr)C and (HfTaTiNbMo)C carbides fabricated through reactive high-energy ball milling and spark plasma sintering. Ceramics International. 2020;46:19008–19014. https://doi.org/10.1016/j.ceramint.2020.04.230
53. Sedegov A.S., Bobojanov A.R., Vorotilo S., Kuskov K.V., Moscovskikh D.O. Synthesis, structure and properties of high entropy materials. IOP Conference Series: Materials Science and Engineering. 2021;1014(1):1–4. https://doi.org/10.1088/1757-899X/1014/1/012049
54. Kovalev D.Yu., Kochetov N.A., Chuev I.I. Fabrication of high-entropy carbide (TiZrHfTaNb)С by high-energy ball milling. Ceramics International. 2021; 47(23):32626–32633. https://doi.org/10.1016/j.ceramint.2021.08.158
55. Mukasyan A.S., Rogachev A.S., Moskovskikh D.O., Yermekova Zh.S. Reactive spark plasma sintering of exothermic systems: A Critical review. Ceramic International. 2022;48(3):2988–2998. https://doi.org/10.1016/j.ceramint.2021.10.207
56. Kochetov N.A., Kovalev I.D. Synthesis and investigation of thermal stability of multicomponent carbide (TaZrHfNbTi)C5. Inorganic Materials. 2021;57(1):8–13. http://dx.doi.org/10.1134/S0020168520120109
57. Vadchenko S.G., Sedegov A.S., Kovalev I.D. Thermal explosions in (Ti, Zr, Hf, Nb, Ta) carbon mixtures. Powder Metallurgy аnd Functional Coatings. 2023;17(3):14–21. https://doi.org/10.17073/1997-308X-2023-3-14-21
58. Vergunova Yu.S., Vadchenko S.G., Kovalev I.D., Kovalev D.Yu., Rogachev A.S., Alymov M.I. Self-propagating high-temperature synthesis of high-entropy carbides in the gasless thermal explosion mode. Doklady Physical Chemistry. 2024;513(1):131–134. https://doi.org/10.1134/S001250162360033X
59. Vadchenko S.G., Kovalev I.D., Mukhina N.I., Sedegov A.S., Rogachev A.S. Thermal Explosion in Ti + Zr + Hf + Nb + Ta + 5C system: effect of mechanical activation. International Journal of Self-Propagating High-Temperature Synthesis. 2022;31(4):207–213. https://doi.org/10.3103/S1061386222040136
60. Evseev N., Matveev A., Belchicov I., Zhukov I. Self-propagating high-temperature synthesis of high-entropy ceramic composition (Hf0.25Ti0.25Cr0.25(FeV)0.25)N. Materials Letters. 2023;346:134562. https://doi.org/10.1016/j.matlet.2023.134562
61. Liu D., Zhang H., You X., Jia J., Meng J. Low temperature synthesized high entropy carbide composites: A potential high temperature anti-wear material. Ceramics International. 2024;50(1):2111–2121. https://doi.org/10.1016/j.ceramint.2023.10.320
62. Potschke J., Vornberger A., Gestrich T., Berger L.-M., Michaelis A., Influence of different binder metals in high entropy carbide based hardmetals. Powder metallurgy. 2022;65(5):373–381. https://doi.org/10.1080/00325899.2022.2076311
63. Luo Si-Chun, Guo Wei-Ming, Lin Hua-Tay. High-entropy carbide-based ceramic cutting tools. Journal American Ceramic Society. 2023;106(2):933–940. https://doi.org/10.1111/jace.18852
64. Patil K.C., Hedge M.S., Rattan T., Aruna S.T. Chemistry of nanocrystalline oxide materials: Combustion synthesis, properties and applications. New Jersey: World Scientific, 2008. 345 p. https://doi.org/10.1142/9789812793157
65. Aiqin Mao, Hou-Zheng Xiang, Zhan-Guo Zhang, Koji Kuramoto, Haiyun Yu, Songlin Ran. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder. Journal of Magnetism and Magnetic Materials. 2019;484:245–252. https://doi.org/10.1016/j.jmmm.2019.04.023
66. Bui L.M., Cam S.T., Buryanenko I.V., Semenov V.G., Nazarov D.V., Kazin P.E., Nevedomskiy V.N., Gerasimov E.Y., Popkov V.I. An ultra-high-entropy rare earth orthoferrite (UHE REO): solution combustion synthesis, structural features and ferrimagnetic behavior. Dalton Transactions. 2023;15:1–3. https://doi.org/10.1039/D2DT04103K
About the Authors
A. R. BobozhanovRussian Federation
Anis R. Bobozhanov – Junior Researcher, Postgraduate Student of the Laboratory of Dynamics of Microheterogeneous Processes
8 Academician Osip’yan Str., Chernogolovka, Moscow Region 142432, Russia
A. S. Rogachev
Russian Federation
Alexander S. Rogachev – Dr. Sci. (Phys.-Math.), Prof., Chief Researcher of the Laboratory of Dynamics of Microheterogeneous Processes
8 Academician Osip’yan Str., Chernogolovka, Moscow Region 142432, Russia
Review
For citations:
Bobozhanov A.R., Rogachev A.S. Self-propagating high-temperature synthesis of high-entropy materials: A review. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):5-16. https://doi.org/10.17073/1997-308X-2024-6-5-16
JATS XML