Scroll to:
Influence of composition and surface roughness of titanium alloys on vital activity of mesenchymal stem cells
https://doi.org/10.17073/1997-308X-2024-6-65-76
Abstract
The study focused on titanium-based alloys for medical applications, including commercially available grades VT1-0 and VT6, and a newly developed alloy with the composition (wt. %): Ti–23Nb–5Zr. The surfaces of all samples underwent sandblasting using six different sand fractions, mechanical grinding, polishing by tumbling, tumbling polishing, and, in the case of the Ti–Nb–Zr alloy, electrolytic plasma polishing. The effects of surface treatment methods and the chemical composition of medical-grade titanium alloys on surface roughness, microhardness, wettability, and interaction with mesenchymal stem cells (MSCs) was investigated. Surface microhardness was measured using the micro-Vickers method with a diamond indenter under varying loads, while surface roughness was determined using a contact profilometer. It was found that electrolytic plasma polishing enhanced both the microhardness and roughness of the alloy compared to tumbling polishing. Wettability was characterized by the contact angle of deionized water, measured using a specialized setup, with the droplet shape described by a 5-point ellipse model. All treated surfaces exhibited wettability; the contact angle increased as surface roughness decreased. However, sandblasting with mixtures containing a wide particle size distribution increased the contact angle due to the more complex surface relief. To evaluate the biological properties of implants made from VT6, VT1-0, and Ti–23Nb–5Zr alloys after the described surface treatments, their effects on cell viability and the adhesive characteristics of the materials were studied using a direct contact method with two types of mesenchymal stem cells. The newly developed alloy, which potentially offers superior biomechanical compatibility compared to commercial materials, demonstrated no compromise in surface characteristics or adverse effects on cell viability.
Keywords
For citations:
Sudarchikova M.A., Nasakina E.O., Davydova G.A., Valiullin L.R., Morozova Ya.A., Kottsov S.Yu., Prokofiev P.A., Konushkin S.V., Sergienko K.V., Sevostyanov M.A., Kolmakov A.G. Influence of composition and surface roughness of titanium alloys on vital activity of mesenchymal stem cells. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):65-76. https://doi.org/10.17073/1997-308X-2024-6-65-76
Introduction
Biomaterials, designed for contact with the living organism’s environment, are commonly used for manufacturing implants. These materials ensure compatibility with medical devices through a combination of properties such as superelasticity, low Young’s modulus, high corrosion resistance, and either bioinertness or adhesiveness [1–3]. Typically, these include metallic alloys (titanium, cobalt, stainless steels), polymers, and ceramics. However, they exhibit certain drawbacks, such as low strength and/or high elastic modulus, which ultimately lead to the degradation of both the implants and surrounding tissues [4–8]. Besides organizational factors (e.g., small-scale production), the manufacturing of titanium-based products is constrained by technological challenges. One such issue is the mechanical strength and fatigue properties of titanium alloy blanks, which, however, can be addressed by developing methods for surface structure modification and optimizing alloy composition.
Shape memory alloys, particularly those in the Ti–Ni system, exhibit low Young’s modulus and superelastic behavior, akin to that of living tissues [9–11]. However, the toxic properties of nickel and the potential for corrosion-related damage (material degradation in operating environments) limit their application [12–14].
At the same time, modern research indicates that shape memory and superelastic effects can also be observed in alloys composed exclusively of non-toxic metals [15–23]. For instance, tantalum [24] and niobium [17; 25–27], which exhibit high corrosion resistance and biocompatibility, can be employed as β-stabilizers in titanium alloys, thereby contributing to a reduction in the elastic modulus. In particular, research [23] has demonstrated that the Ti–Nb–Ta alloy has a lower elastic modulus and higher corrosion resistance compared to the Ti–6Al–4V alloy. Zirconium is typically used as a neutral strengthening element [28–31]; however, as reported by the authors of [20], it may also exhibit β-stabilizing effects in β-titanium alloys. Furthermore, titanium and niobium have similar atomic radii (0.145–0.146 nm), whereas zirconium has a slightly larger atomic radius (0.160 nm). Thus, alloying titanium with zirconium is expected to increase the interatomic distance in the alloy, reduce the bonding force between atoms, and consequently lower the Young’s modulus. Conversely, alloying titanium with niobium is expected to at least maintain the lattice parameter of the β-phase.
Therefore, Ti–Nb–Zr alloys can be considered excellent candidates for biomedical applications involving implantation.
Mesenchymal stem cells (MSCs) are an optimal test system for analyzing the biological activity of materials intended for implant fabrication, as they possess a high potential for differentiation into cellular elements of various mesenchymal-derived tissues [32].
The interaction of any material with cells is largely influenced by the quality of its surface. Therefore, the aim of this study was to investigate the interaction of the Ti–Nb–Zr alloy, following various surface treatments, with mesenchymal stem cells in comparison with materials already used in medical applications.
Materials and methods
Samples for the study were fabricated from the following titanium-based biomedical materials:
– commercially available material VT1-0 (pure titanium), as per GOST 19807-91;
– commercially available alloy VT6 (titanium alloy with aluminum and vanadium), as per GOST 19807-91;
– a newly developed titanium-based alloy with the composition (at. %): Ti–23Nb–5Zr.
The starting materials used included iodide titanium, niobium grade Nb-1, and iodide zirconium. Alloy ingots were melted in an argon-arc melting furnace with a non-consumable tungsten electrode. To produce sheets, the ingots underwent homogenizing annealing in a vacuum, followed by warm rolling with intermediate annealing and subsequent quenching.
The Young’s modulus of the Ti–23Nb–5Zr alloy sheet samples was determined using a universal testing machine, INSTRON 3382 (USA), at room temperature.
The interaction of any material with cells depends significantly on the quality of its surface; hence, several surface treatment options were selected for evaluation. Samples of the VT1-0, VT6, and Ti–23Nb–5Zr alloys, with a diameter of 20 mm and thickness of 4 mm, were cut from sheets using a DK 7745 ME11 electrical discharge machine by Meatec (China). Before sandblasting, the samples were pre-ground using abrasive papers with grit sizes ranging from 240 to 600 and treated by tumbling in a KT-100 electromagnetic tumbling machine (CARLO de GIORGI, Italy) with a metallic needle abrasive. Additionally, some samples were subjected to electrolytic plasma polishing (EPP) in a 5 % aqueous solution of a 20 % NH4F + 80 % KF mixture at a voltage of 300 V and a temperature of 85–88 °C for 10 min. The sandblasting of sample surfaces was conducted in a 90 L chamber under 12 atm of high-purity argon pressure using copper slag (particle sizes ≤0.63 mm) and sand fractions of 0.63–1.0 mm, 1.0–1.5 mm, and 0.63–1.5 mm, as well as their 1:1 mixture (0.63–1.5 mm). For the treated surfaces, characteristics such as roughness and wettability were determined.
Surface roughness was evaluated according to GOST 25142-82 using a Proton profilometer model 130 (Russia). Prior to measurement, all samples were cleaned in an ultrasonic bath with a specialized soap solution, bidistilled water, and alcohol, then carefully dried.
Surface microhardness (HV) was determined using the micro-Vickers method as per GOST 9450-76 with a 401/402-MVD device (Wolpert Group, Germany) equipped with an optical microscope. A diamond indenter with a tip size of 10 µm and test loads of 25, 100, 300, and 500 g were used.
The wettability of the samples was characterized by the contact angle of deionized water, measured using a Lonroy SDC-350 setup (Dongguan Lonroy Equipment Co., LTD, China) at a tilt angle of 0°. A droplet with a volume of 6 µL was deposited on the sample, and an image of the droplet was taken 60 s later. When measuring the contact angle, the droplet shape was analyzed after a delay following its contact with the substrate. This delay helps eliminate dynamic effects that could distort the shape of the elastic droplet immediately upon impact with the sample. Typically, the waiting time before droplet shape analysis is 30–60 s after the start of the measurement [33–35]. When calculating the contact angle, the droplet shape was described using a 5-point ellipse model.
To evaluate the biological properties of implants made from VT6, VT1-0, and Ti–23Nb–5Zr alloys following the described surface treatments, as well as the effect of the samples on cell viability and the adhesive characteristics of the materials, a direct contact method was employed using two types of mesenchymal stem cells (MSCs): dental pulp stem cells (DPSCs) from human dental pulp (clone Th44), and immortalized fibroblast-like cells (embryonic connective tissue cells involved in regeneration and synthesis of proteins critical for dermal rejuvenation [36], skin MSCs [37]).
For cell viability assessment, the samples were sterilized with 70 % ethanol and placed in wells of a 24-well plate. DPSC cells at the 5th passage were seeded into the plate wells at a density of 30,000 cells/cm2 in DMEM/F12 medium containing 10 % FBS and supplemented with 100 U/mL penicillin/streptomycin. The cells were cultured for 24 h at 37 °C in a humidified atmosphere with 5 % CO2 . Following the culture period, the morphology of the cells in direct contact with the samples was assessed on the surface of the culture plastic.
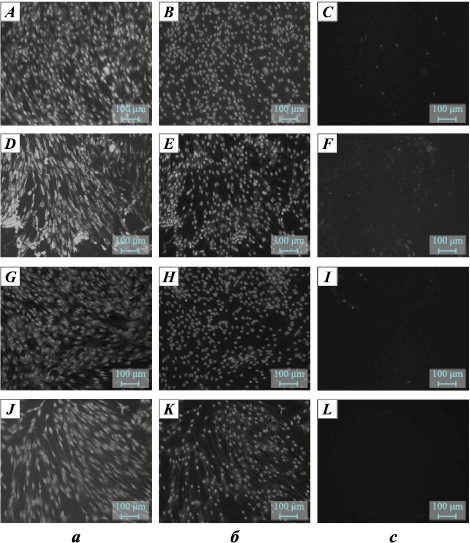
As a negative control, DMEM/F12 medium without cells was added to the wells. At the end of the cultivation period, the morphology of the cells on the surfaces of the tested materials was evaluated, and cell viability was assessed using fluorescent staining with SYTO 9, propidium iodide (PI), and Hoechst 33342 reagents. The fluorescent dye SYTO 9, at an excitation wavelength (λexc ) of 450÷490 nm and an emission wavelength (λem ) of 515÷565 nm, stains the DNA and RNA of both live and dead cells green. The intercalating reagent propidium iodide (PI), with λexc = 546 nm and λem = 575÷640 nm, stains the nuclei of dead cells red. The fluorescent dye Hoechst 33342, with λexc = 343 nm and λem = 483 nm, stains the DNA of both live and dead cells blue.
The effect of the tested materials on fibroblast cells was evaluated by culturing the cells directly in the presence of the samples. After 24 h, the cell layer was evaluated using an inverted microscope based on the following parameters: surface coverage area, cell shape, the number of cellular aggregates, and the number of floating cells. Cell counting was performed using a Goryaev chamber, and the number of viable and dead cells was determined using trypan blue staining (0.1 % solution) [38]. The influence of the Ti-based samples on the culture morphology of the cells was determined based on the following metrics:
– viability coefficient: the ratio of live cells to the total number of cells, %;
– proliferation index: the ratio of the number of grown cells to the number of seeded cells [39];
– cell death percentage: the ratio of the number of dead cells remaining after exposure to the compound to the total number of cells after exposure, %.
Statistical analysis of the obtained data was conducted using the methods of variation statistics with Student’s t-test to assess significance.
The isolation of dental pulp stem cells (DPSCs) was performed as follows: after opening the crown, the pulp was extracted, washed with Hank’s solution, minced, and incubated in a 0.1 % type I collagenase solution for 30 min at 37 °C. The resulting cell suspension was centrifuged at 1000 rpm for 5 min. The pellet was resuspended in growth medium (DMEM/F12) supplemented with 10 % fetal calf serum (FCS), 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM glutamine, and then transferred into culture flasks. After 3 days, non-adherent cells were removed, and the fraction of adherent cells was cultured until 80–90 % confluence was reached. The cells were then suspended using a mixture of 0.25 % trypsin solution and Versene solution (1:1) and reseeded at a 1:3 ratio. Passaging of cells in-vitro was performed using standard methods in culture flasks within a CO2 incubator (37 °C, 5 % CO2 , 80 % humidity) with the growth medium changed every 3 days.
Results and discussion
The Young’s modulus of the Ti–23Nb–5Zr alloy surface in its initial state was Е = 56±5 GPa, which is significantly lower than that of the commercially available alloys VT1-0 and VT6 (E > 90 GPa) [40; 41] and is closer to the values of bone [42].
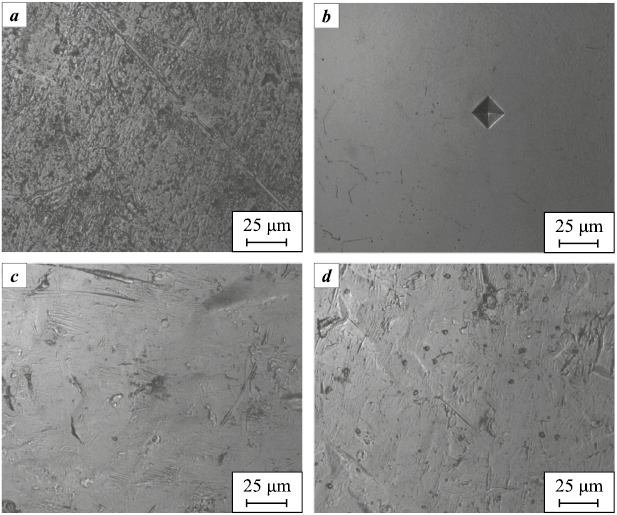
Six types of samples were prepared for each alloy, differing in the method of sandblasting and the presence of electrolytic plasma polishing (EPP). The results of roughness (Ra ) and microhardness (HV) measurements for the samples are presented in Tables 1 and 2, while microphotographs of the polished samples (tumbling and EPP) are shown in Fig. 1. High surface heterogeneity after sandblasting prevented reliable results due to indenter slippage. The Ra values for the samples after tumbling were lower than those after EPP. This can be explained by the fact that, in the first case, a kind of “smoothing” of the surface relief occurs, while in the latter, preferential etching of certain structural components and segregation zones may take place. No correlation was observed between the roughness and microhardness of the samples.
Table 1. Results of roughness and Vickers microhardness at 500 g load,
Table 2. Results of studies of microhardness of polished samples depending on the load
Fig. 1. Microphotographs of samples with smooth surfaces | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
At the same time, electrolytic plasma polishing contributes to an increase in the hardness of the surface layer (see Tables 1 and 2). This is presumably due to structural changes in the surface layer of the metallic materials, which influence their mechanical properties [43]. Notably, as the load decreases, microhardness increases, since the contribution of the surface itself becomes more significant compared to the bulk material (Table 2). No correlation was observed between surface roughness and microhardness of the samples.
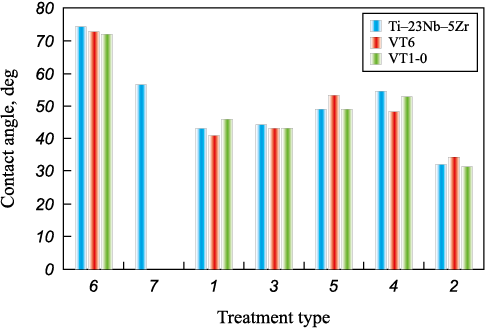
The effect of treatment on surface roughness is consistent with the results of contact angle measurements (see Table 1, Fig. 2). A typical trend observed for solid materials wetted by liquid is that the contact angle increases (wettability decreases) as surface roughness decreases [44]. Minimum wettability was observed after tumbling. However, a deviation from the direct relationship occurred for all materials treated with a mixture of copper slag and original (non-fractionated) sand. This is likely due to the wide range of particle sizes impacting the surface, resulting in many small cavities among the larger ones, which locally increased the contact angle. For a similar reason, albeit to a lesser extent, the contact angle for samples treated with original sand also deviates from the trend, as the range of particle sizes is narrower. All three alloys demonstrated similar surface characteristics after each type of treatment.
Fig. 2. Dependence of wettability on surface treatment (see Table 1) |
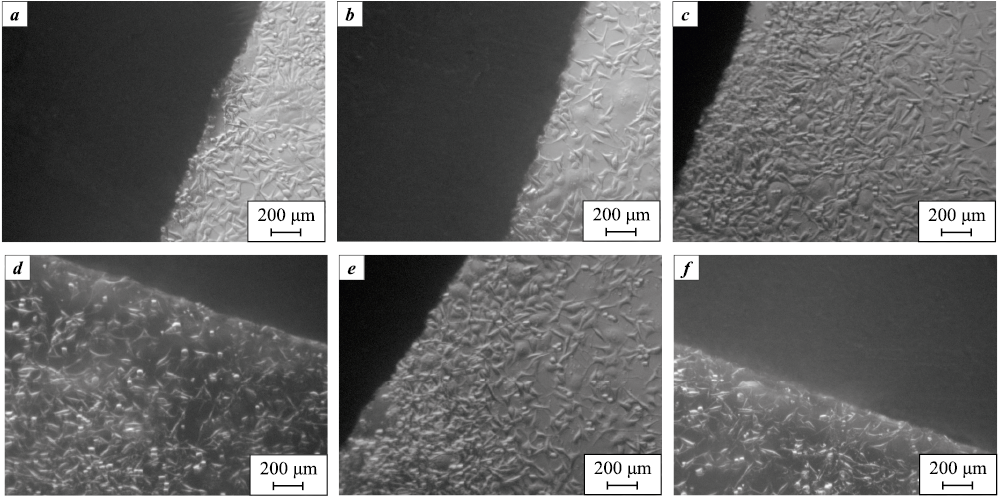
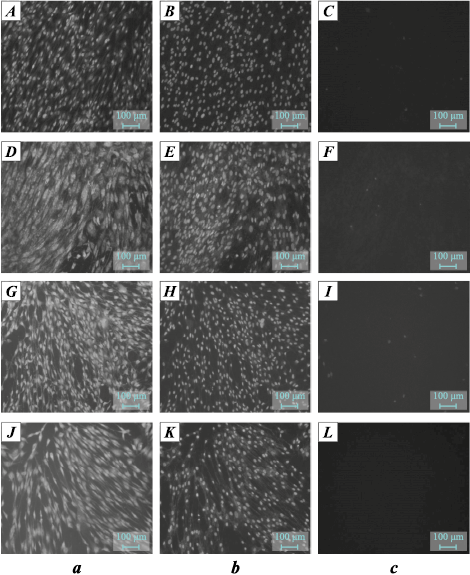
The images in Fig. 3 show the appearance of DPSC cells at the contact area with the tested materials after 24 h of cultivation. It is evident that none of the materials inhibit cell growth, demonstrating their biocompatibility1. No differences were observed depending on the alloy composition or treatment type.
Fig. 3. Appearance of DPSC cells at the contact area with tested materials 24 h after seeding |
Characteristic microphotographs from the first day of cultivation for samples treated with copper slag and sand (1.0–1.5 mm) are shown in Fig. 4 and 5, respectively. The surfaces displayed a large number of “spread-out” cells, evenly distributed with only a few non-viable ones. This demonstrates that all sample types were adhesive to cells.
Fig. 4. Appearance of DPSC cells incubated on the surface
Fig. 5. Appearance of DPSC cells incubated on the surface |
Table 3 presents the culture-morphological properties of fibroblast cells in contact with the alloys after sandblasting treatments. All materials showed low cytotoxicity, highlighting their suitability for medical applications. No correlation was found between the cytotoxic properties of the alloys and their composition or surface treatment methods.
Таблица 3. Cytotoxic properties of alloys depending on surface treatment
|
Conclusion
The study examined the effects of six sandblasting techniques and electrolytic plasma polishing (EPP) on the surface properties and interaction with mesenchymal stem cells of two commercially available titanium-based alloys – VT1-0 (pure titanium) and VT6 (a titanium-aluminum-vanadium alloy) – as well as a newly developed Ti–Nb–Zr alloy.
EPP was found to enhance microhardness but reduce surface roughness compared to tumbling.
All treated surfaces exhibited wettability, with the contact angle increasing as surface roughness decreased. However, sandblasting with mixtures containing a wide range of particle sizes increased the contact angle, likely due to the creation of a more complex surface texture. Tumbling produced the highest contact angle among all alloys, resulting in the most “developed” surface relief.
All three materials exhibited low cytotoxicity, high proliferative activity, and excellent cell viability. A significant number of viable cells were evenly distributed on the sample surfaces. No definitive relationship was observed between cytotoxicity parameters and either the material composition or the surface treatment method.
The newly developed alloy Ti–23Nb–5Zr, which shows promise for improved biomechanical compatibility compared to the commercial alloys, demonstrated stable surface properties and had no adverse effects on cell viability.
References
1. Park J.B., Lakes R.S. Metallic implant materials. Biomaterials. 2007;5:99–137. https://doi.org/10.1007/978-0-387-37880-0_5
2. Amirtharaj Mosas K.K., Chandrasekar A.R., Dasan A., Pakseresht A., Galusek D. Recent advancements in materials and coatings for biomedical implants. Gels. 2022;8(5):323. https://doi.org/10.3390/gels8050323
3. Pandey A., Awasthi A., Saxena K.K. Metallic implants with properties and latest production techniques: A review. Advances in Materials and Processing Technologies. 2020;6(2):405–440. https://doi.org/10.1080/2374068X.2020.1731236
4. Biesiekierski A., Wang J., Gepreel M. A.-H., Wen C. A new look at biomedical Ti-based shape memory alloys. Acta Bio-Materialia. 2012;8(5):1661–1669. https://doi.org/10.1016/j.actbio.2012.01.018
5. Zhao X., Zhang J., Song X., Guo W. Investigation on mechanical properties of laser welded joints for Ti–6Al–4V titanium alloy. Materials Science and Technology. 2013;29(12):1405–1413. https://doi.org/10.1179/1743284713Y.00000003
6. Nune K.C., Misra R., Gai X., Li S.J., Hao Y.L. Surface nanotopography-induced favorable modulation of bioactivity and osteoconductive potential of anodized 3D printed Ti–6Al–4V alloy mesh structure. Journal of Biomaterials Applications. 2018;32(8):1032–1048. https://doi.org/10.1177/0885328217748860
7. Navarro M., Michiardi A., Castaño O., Planell J.A. Biomaterials in orthopaedics. Journal of the Royal Society Interface. 2008;5(27):1137–1158. https://doi.org/10.1098/rsif.2008.0151
8. Renganathan G., Tanneru N., Madurai S. L. Orthopedical and biomedical applications of titanium and zirconium metals. Fundamental Biomaterials: Metals. 2018:211–241. https://doi.org/10.1016/B978-0-08-102205-4.00010-6
9. Alferi D., Fojt J., Kristianova E., Edwards D.W., Laasch H.-U. Influence of the manufacturing process on the corrosion and mechanical behavior of Esophageal stents. Metals. 2023;13(9):1542. https://doi.org/10.3390/met13091542
10. Elsisy M., Chun Y. Materials properties and manufacturing processes of nitinol endovascular devices. In: Bártolo, P.J., Bidanda, B. (eds) Bio-materials and prototyping applications in medicine. Springer, Cham. 2021. P. 59–79. https://doi.org/10.1007/978-3-030-35876-1_4
11. Stoeckel D., Bonsignore C., Duda S. A survey of stent designs. Minimally Invasive Therapy & Allied Technologies. 2002;11(4):137–147. https://doi.org/10.1080/136457002760273340
12. Nasakina E.O., Sudarchikova M.A., Sergienko K.V., Konushkin S.V., Sevost’yanov M.A. Ion release and surface characterization of nanostructured nitinol during long-term testing. Nanomaterials. 2019;9(11):1569. https://doi.org/10.3390/nano9111569
13. Zhang Y., Zhang Z.W., Xie Y.M., Wang S.S., Qiu Q.H., Zhou Y.L., Zeng G.H. Toxicity of nickel ions and comprehensive analysis of nickel ion-associated gene expression profiles in THP-1 cells. Molecular Medicine Reports. 2015;12(3):3273–3278. https://doi.org/10.3892/mmr.2015.3878
14. Lü X., Bao X., Huang Y., Qu Y., Lu H., Lu Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials. 2009;30(2):141–148. https://doi.org/10.1016/j.biomaterials.2008.09.011
15. Chen L.Y., Cui Y.W., Zhang L.C. Recent development in beta titanium alloys for biomedical applications. Metals. 2020;10(9):1139. https://doi.org/10.3390/met10091139
16. Dobromyslov A.V., Elkin V.A. Martensitic transformation and metastable β-phase in binary titanium alloys with d-metals of 4–6 periods. Scripta Materialia. 2001;44(6):905–910. https://doi.org/10.1016/S1359-6462(00)00694-1
17. Kim H.Y., Hashimoto S., Kim J.I., Inamura T., Hosoda H., Miyazaki S. Effect of Ta addition on shape memory behavior of Ti–22Nb alloy. Materials Science and Engineering: A. 2006;417(1-2):120–128. https://doi.org/10.1016/j.msea.2005.10.065
18. Weinmann M., Schnitter C., Stenzel M., Markhoff J., Schulze C., Bader R. Development of bio-compatible refractory Ti/Nb(/Ta) alloys for application in patient-specific orthopaedic implants. International Journal of Refractory Metals and Hard Materials. 2018;75:126–136. https://doi.org/10.1016/j.ijrmhm.2018.03.018
19. Miyazaki S., Kim H. Y., Hosoda H. Development and characterization of Ni-free Ti-base shape memory and superelastic alloys. Materials Science and Engineering: A. 2006;438–440:18–24. https://doi.org/10.1016/j.msea.2006.02.054
20. Guehennec L. Surface treatments of titanium dental implants for rapid osseointegration. Dental Materials. 2007;23(7):844–854. https://doi.org/10.4012/dmj.2012-015
21. Dubinskiy S.M., Prokoshkin S.D., Brailovski V., Inaekyan K.E., Korotitskiy A.V., Filonov M.R., Petrzhik M.I. Structure formation during thermomechanical processing of Ti-Nb-(Zr, Ta) alloys and the manifestation of the shape-memory effect. The Physics of Metals and Metallography. 2011;112(5):503–516. https://doi.org/10.1134/S0031918X11050206
22. Liu J., Chang L., Liu H., Li Y., Yang H., Ruan J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb–Ti–Ta alloys as biomedical material. Materials Science and Engineering: C. 2017;71:512–519. https://doi.org/10.1016/j.msec.2016.10.043
23. Hussein A.H., Gepreel M.A.H., Gouda M.K., Hefnawy A.M., Kandil S.H. Biocompatibility of new Ti–Nb–Ta base alloys. Materials Science and Engineering: C. 2016;61: 574–578. https://doi.org/10.1016/j.msec.2015.12.071
24. Zhou Y.L., Niinomi M., Akahori T. Effects of Ta content on Young’s modulus and tensile properties of binary Ti–Ta alloys for biomedical applications. Materials Science and Engineering: A. 2004;371(1-2):283–290. https://doi.org/10.1016/j.msea.2003.12.011
25. Cheng X., Liu S., Chen C., Chen W., Liu M., Li R., Zhang X., Zhou K. Microstructure and mechanical properties of additive manufactured porous Ti–33Nb–4Sn scaffolds for orthopaedic applications. Journal of Materials Science: Materials in Medicine. 2019;30:1–12. https://doi.org/10.1007/s10856-019-6292-0
26. Li Y.H., Shang X.Y. Recent progress in porous TiNb-based alloys for biomedical implant applications. Materials Science and Technology. 2020;36(4):385–392. https://doi.org/10.1080/02670836.2020.1724415
27. Zhao D., Chang K., Ebel T., Qian M., Willumeit R., Yan M., Pyczak F. Microstructure and mechanical behavior of metal injection molded Ti–Nb binary alloys as biomedical material. Journal of the Mechanical Behavior of Biomedical Materials. 2013;28:171–182. https://doi.org/10.1016/j.jmbbm.2013.08.013
28. Munir K., Lin J., Wright P. F., Ozan S., Li Y., Wen C. Mechanical, corrosion, nanotribological, and biocompatibility properties of equal channel angular pressed Ti–28Nb–35.4 Zr alloys for biomedical applications. Acta Biomaterialia. 2022;149:387–398. https://doi.org/10.1016/j.actbio.2022.07.005
29. Li S., Choi M., Nam T. Effect of thermo-mechanical treatment on microstructural evolution and mechanical properties of a superelastic Ti–Zr-based shape memory alloy. Materials Science and Engineering: A. 2020;789:139664. https://doi.org/10.1016/j.msea.2020.139664
30. Hussein M. A., Kumar M., Drew R., Al-Aqeeli N. Electrochemical corrosion and in vitro bioactivity of nano-grained biomedical Ti–20Nb–13Zr alloy in a simulated body fluid. Materials. 2017;11(1):26. https://doi.org/10.3390/ma11010026
31. Kim J. I., Kim H. Y., Inamura T., Hosoda H., Miyazaki S. Shape memory characteristics of Ti–22Nb–(2–8) Zr (at.%) biomedical alloys. Materials Science and Engineering: A. 2005;403(1-2):334–339. https://doi.org/10.1016/j.msea.2005.05.050
32. Lykov A.P. Mesenchymal stem cells: properties and clinical application. Sibirskiy nauchnyi meditsinskiy zhurnal. 2023;43(2):40–53. (In Russ.). https://doi.org/10.18699/SSMJ20230204
33. Lanning B.R. Method for predicting wettability and interfacial bond strengths at metal/ceramic interfaces. Mines Theses & Dissertations. Ann Arbor: ProQuest LLC, 1990. 180 p.
34. Hebbar R.S., Isloor A.M., Ismail A.F. Chapter 12 – Contact angle measurements. In: Membrane characterization. Massachusetts: Elsevier Inc., 2019. P. 219–255. https://doi.org/10.1016/B978-0-444-63776-5.00012-7
35. Decker E.L., Frank B., Suo Y., Garoff S. Physics of contact angle measurement. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1999;156(1-3): 177–189. https://doi.org/10.1016/S0927-7757(99)00069-2
36. Shurygina I.A., Shurygin M.G., Ayushinova N.I., Kanya O.V. Fibroblasts and their role in the development of connective tissue. Baykal’skiy meditsinskiy zhurnal. 2012;110(3):8–12. (In Russ.).
37. Pavlova S.V., Sergeyevichev D.S., Chepeleva Ye.V., Kozyreva V.S., Malakhova A.A., Zakharova I.S., Zakiyan S.M. Comparison of mesenchymal stromal cells of the bone marrow and regional stem cells of the heart and human skin fibroblasts. Patologiya krovoobrashcheniya i kardiokhirurgiya. 2015;19(S4-2):12–19. (In Russ.).
38. Nabatov A.A., Raginov I.S. The DC-SIGN-CD56 interaction inhibits the anti-dendritic cell cytotoxicity of CD56 expressing cells. Infectious Agents and Cancer. 2015(10):1–11. https://doi.org/10.1186/s13027-015-0043-8
39. Dyakonov L.P., Akinshina G.T., Bilko N.M., Galnbek T.V. Animal cell in culture. Ed. 2nd updated. Moscow: Companiya Sputnik, 2009. 652 p. (In Russ.).
40. Lutfullin R.Ya., Trofimova Ye.A. Young’s modulus of titanium alloy VT6S and its structural sensitivity. Letters on Materials. 2017;7(1):12–16. (In Russ.). https://doi.org/10.22226/2410-3535-2017-1-12-16
41. GOST R 8.982-2019. Titanium alloys grade VT. Sound velocity, relative thermal expansion, density and Young’s modulus in the temperature range from 20 °C to 800 °C. Moscow: Standartinform, 2019. 20 p. (In Russ.).
42. Wu D., Isaksson P., Ferguson S.J., Persson C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomaterialia. 2018;78:1–12. https://doi.org/10.1016/j.actbio.2018.08.001
43. Terent’yev V.F., Slizov A.K., Smyslov A.M., Tamidarov D.R., Prosvirnin D.V., Penkin A.G., Shiryayev L.P., Sirotinkin V.P. Effect of electrolytic-plasma polishing on the mechanical properties of austenitic–martensitic VNS9-Sh TRIP steel. Russian Metallurgy (Metally). 2020;2020(10):1199–1206. https://doi.org/10.1134/S0036029520100286
44. Grodsky A.S., Kienskaya K.I., Gavrilova N.N., Nazarov V.V. Basic concepts and equations of colloidal chemistry. Moscow: RСTU named after D.I. Mendeleeva, 2013. 40 р. (In Russ.).
About the Authors
M. A. SudarchikovaRussian Federation
Maria A. Sudarchikova – Junior Research Scientist, Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
E. O. Nasakina
Russian Federation
Elena O. Nasakina – Cand. Sci. (Eng.), Senior Research Scientist, Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
G. A. Davydova
Russian Federation
Galina A. Davydova – Cand. Sci. (Phys.-Math.), Leading Research Scientist, Laboratory of Cell and Tissue Growth
3 Institutskaya Str., Pushchino, Moscow region 142290, Russia
L. R. Valiullin
Russian Federation
Lenar R. Valiullin – Cand. Sci. (Biolog.), Head of the Laboratory of Probiotic Preparations and Enzymes
2 Nauchny Town, Kazan, Republic of Tatarstan 420075, Russia
Ya. A. Morozova
Russian Federation
Yaroslava A. Morozova – Research Engineer, Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
S. Yu. Kottsov
Russian Federation
Sergey Yu. Kottsov – Junior Research Scientist, Laboratory of Synthesis of Functional Materials and Processing of Mineral Raw Materials
31 Leninskiy Prosp., Moscow 119071, Russia
P. A. Prokofiev
Russian Federation
Pavel A. Prokofiev – Cand. Sci. (Eng.), Junior Research Scientist, Laboratory of Physicochemistry of Refractory and Rare Metals and Alloys
49 Leninskiy Prosp., Moscow 119334, Russia
S. V. Konushkin
Russian Federation
Sergey V. Konushkin – Cand. Sci. (Eng.), Research Scientist of the Laboratory of Strength and Plasticity of metal and composite materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
K. V. Sergienko
Russian Federation
Konstantin V. Sergienko – Junior Research Scientist, Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
M. A. Sevostyanov
Russian Federation
Mikhail A. Sevostyanov – Cand. Sci. (Eng.), Leading Research Scientist, Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
A. G. Kolmakov
Russian Federation
Alexey G. Kolmakov – Dr. Sci. (Eng.), Corresponding Member of the Russian Academy of Sciences, Head of the Laboratory of Strength and Plasticity of Metal and Composite Materials and Nanomaterials
49 Leninskiy Prosp., Moscow 119334, Russia
Review
For citations:
Sudarchikova M.A., Nasakina E.O., Davydova G.A., Valiullin L.R., Morozova Ya.A., Kottsov S.Yu., Prokofiev P.A., Konushkin S.V., Sergienko K.V., Sevostyanov M.A., Kolmakov A.G. Influence of composition and surface roughness of titanium alloys on vital activity of mesenchymal stem cells. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2024;18(6):65-76. https://doi.org/10.17073/1997-308X-2024-6-65-76