Scroll to:
Functional coatings of submersible oilfield equipment for protection against corrosion, asphalt, resin, paraffin and salt deposits: Review
https://doi.org/10.17073/1997-308X-2025-1-58-74
Abstract
The oil production process is often accompanied by various failures at oil production facilities, which leads to serious economic losses. Failures in oil production systems not only increase repair and maintenance costs, but also lead to loss of productivity, which has a negative impact on the economic efficiency of projects. A pipeline failure is considered to be its complete or partial shutdown due to a violation of its tightness or tightness of the shut-off valves, or due to blockage of the flow section. The most common causes of complications in oil production are: corrosion of oil and gas equipment, formation of asphalt-resin-paraffin deposits (ARPD) and inorganic salt deposits on the working surface of oil and gas equipment. There are a large number of methods aimed at preventing each of the previously mentioned complicating factors. It is noteworthy that the use of protective coatings can be a measure of prevention of corrosion processes, ARPD, and inorganic salt deposits. This article will review the literature, which will consider what properties, composition and structure protective coatings should have to prevent corrosion, ARPD and salt deposits, as well as what testing methods can be used to evaluate the ability of a protective coating to prevent these complicating factors.
Keywords
For citations:
Yudin P.E. Functional coatings of submersible oilfield equipment for protection against corrosion, asphalt, resin, paraffin and salt deposits: Review. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(1):58-74. https://doi.org/10.17073/1997-308X-2025-1-58-74
Introduction
Oil and gas fields are gradually entering the late stage of development, which is accompanied by increasing complexity in operational processes [1; 2]. At this stage, the risks of equipment failures rise significantly due to a number of factors specific to this phase of the field’s lifecycle. Various complicating factors act jointly and interdependently at oilfields. However, each well typically has one dominant complication type, which serves as the primary cause of most failures. The distribution of so-called complicated wells across different production companies by their main complication type provides valuable insights into the scale and actual prevalence of corrosion-related issues within the industry.
The structure of complicated wells at LLC RN-Purneftegaz includes only 18 % of wells prone to the formation of asphalt-resin-paraffin deposits (ARPD), 13 % of wells where failures are caused by salt deposits, and only 6 % of wells where corrosion is the primary complication. It is important to note that the number of failures related to corrosion significantly decreased only after the implementation of corrosion-resistant pipes; however, it still remains high [3]. At Udmurtneft PJSC, corrosion accounts for 39 % of well failures [4]. A substantial number (26 %) of Udmurtneft’s wells are affected by ARPD, while 1 % of the wells experience complications due to inorganic salt deposits [4]. As of early 2022, the complicated well stock of PJSC Lukoil included 14,271 wells, representing 45 % of all operating artificial lift wells [5]. The structure of Lukoil’s complicated well stock consists of 74.8 % of wells affected by ARPD, 9.5 % by corrosion, and 3.8 % by inorganic salt deposits [5].
Corrosion is a fundamental issue in any industry dealing with chemically active environments. In oil and gas production, its consequences include the irreversible loss of pipe metal [6], costs associated with equipment replacement, lost profits due to well downtime during repairs, as well as expenses related to mitigating accident consequences and maintenance of corrosion protection systems. All these factors increase production costs and reduce field profitability.
Globally, corrosion results in substantial direct and indirect financial losses [7–9]. In Russia, annual metal losses due to corrosion amount to 12 % of the country’s total metal reserves, which is equivalent to the same share of the annual steel production. Approximately 10 million tons out of the 70 million tons produced annually are lost to corrosion, which translates into financial losses of USD 8 billion. Nationwide, 400,000–500,000 tons of steel are used annually to replace various types of pipelines [6].
Complications associated with the formation of ARPD [10–13] and inorganic salt deposits are no less significant, as they lead to partial or complete blockages of the internal pipe cross-section, resulting in reduced production rates or even cessation of oil extraction [14–16].
The most effective solution to combat these types of complications today is the application of various functional coatings – polymeric, ceramic, and metallization – depending on the type of equipment being protected [17].
This article reviews the application of various functional coatings for oil and gas equipment aimed at counteracting complicating factors.
Functional internal coatings for tubing
Tubing (TBG) is one of the key components of submersible oilfield equipment, susceptible to corrosion, asphalt-resin-paraffin deposits (ARPD), and salt deposits on its inner surface, which is in direct contact with the extracted medium. The housings of submersible electric motors (SEM) and electric submersible pumps (ESP) are exposed to the extracted media from their external surface. It should be noted that cases of contact between the medium and the external surface of tubing are possible; however, on the one hand, such occurrences are rare, and on the other hand, the only available method of protection against corrosion in such cases is the use of alloyed corrosion-resistant steels.
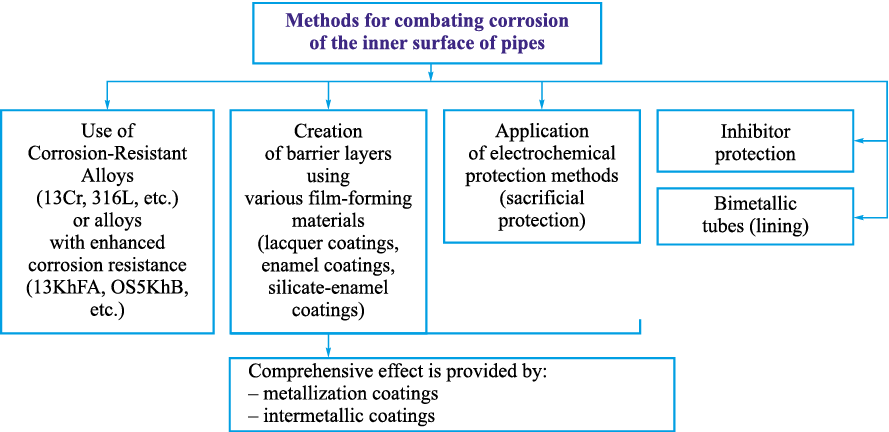
Currently, there is no established classification system for internal tubing corrosion protection methods. Therefore, the author has proposed a classification (Fig. 1) that summarizes various approaches – ranging from traditional steel alloying to the development of duplex coatings [18; 19], which combine sacrificial and barrier properties.
Fig. 1. Classification of methods for combating corrosion on the inner surface of tubing strings |
Austenitic stainless steel tubing is not manufactured due to its extremely high cost combined with relatively low strength properties [20]. However, this class of steel has found its application in the production of liners (thin-walled internal metal tubes) used in bimetallic pipes [21]. The installation of liners in production and tubing pipes can be performed using several methods; however, currently, only two methods have been implemented in Russia [22–24]. In both cases, the first step involves inserting a liner with a diameter smaller than the internal diameter of the tubing. The liner is secured by mechanical interference through roller expansion [25] or by a hydraulic method [26]. In both methods, the gap between the liner and the tubing is eliminated, and the resulting interference fit ensures a stable liner position under all permissible operational loads. In large-diameter pipes (used for trunk oil and gas pipelines), a metallurgical method is applied, forming a diffusion transition zone between the metals.
Various austenitic stainless steels with different chemical compositions can be used for this technology. The most commonly used steels are AISI 304, being the most cost-effective in this class; however, their corrosion resistance is often insufficient for highly mineralized environments. In such cases, more highly alloyed steels such as AISI 316, 316L, or 825 are required.
The advantages of this technology include the relatively low cost of tubing with liners compared to pipes made entirely from liner material; the ability to achieve near-absolute corrosion resistance by selecting an appropriate liner material; and the absence of temperature limitations typical for polymer coatings. However, the disadvantages of this technology include higher costs compared to low-alloy steel pipes, low efficiency if the liner material is improperly selected, lack of reliable methods for protecting the annular space of couplings at pipe ends, and an increase in tubing string weight by 8–11 %.
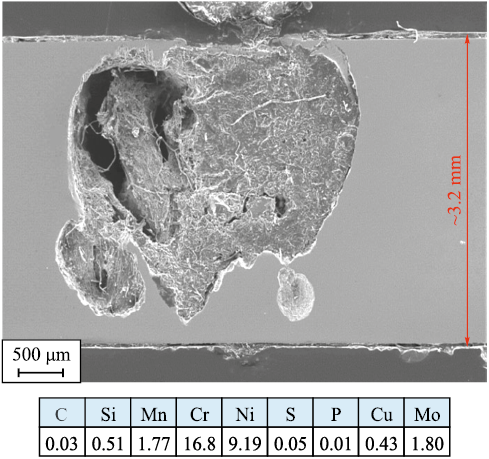
It is also important to highlight the risk of pitting corrosion in austenitic steels when exposed to environments with high chloride ion (Cl\(^–\)) content. Fig. 2 shows an example of the operation of a tubular component made from AISI 316 steel as part of a pipeline transporting seawater. The corrosion rate of the component was 12.8 mm/year. This phenomenon is most likely to occur due to β-phase precipitation, such as during cold plastic deformation. Before operating in such environments, it is mandatory to conduct pitting corrosion resistance testing in ferric chloride solution according to GOST 9.912–89.
Fig. 2. Longitudinal section of the tubular component wall made of AISI 316 steel |
Bimetallic pipes with an internal liner can be considered a specialized type of coated pipe, with the liner serving as a protective barrier. The mechanism by which the liner provides protection is similar to that of conventional coatings, resulting in comparable performance requirements for both. Based on an analysis of liner steel corrosion, manufacturing technologies, and operational factors, GOST 70926–2023, “Tubing with Internal Liner. Technical Specifications”, was developed under the author’s supervision. Pipes produced in accordance with this standard have been in trouble-free operation across Russia for more than three years. Their use commenced even before the official publication of GOST 70926–2023, following the completion of research and development activities that formed the basis for the standard. Condition monitoring has shown no signs of corrosion damage to the liner. The service life of these pipes is primarily constrained by factors such as length reduction due to repeated thread cutting, external surface corrosion, and mechanical damage.
The most commonly used corrosion-resistant steels are alloyed with chromium in concentrations of 13 % or more. According to Schaeffler’s rule, their protection mechanism is based on the formation of a passive chromium oxide film on the surface, which is resistant to interaction with corrosive gases dissolved in the produced fluid. Traditionally, Cr13-grade steel pipes are considered the benchmark for corrosion resistance; however, their widespread adoption is limited by their high cost. Certain factors significantly reduce the service life of such pipes and lead to premature failure, all of which are associated with the degradation of the passive layer. Since the produced fluid lacks oxygen, repassivation is not possible. The operational limitations of Cr13-grade pipes include the following: acid treatments, mechanical wear (including erosion), and cable insulation failure. In the presence of hydrogen sulfide in the well and acid treatments, sulfide stress corrosion cracking (SSCC) may occur [27; 28], which is typical for low-alloy steels with a hardness exceeding 22 HRC.
The term “steel with enhanced corrosion resistance” was introduced in Russia in the mid-2000s. This class includes steels alloyed with 0.5–1.0 % Cr, often with the addition of niobium or vanadium to refine the grain structure, as well as copper. However, according to GOST 5272–50, these steels are classified as low-alloy and are not considered corrosion-resistant (it is worth noting that the later and currently relevant edition, GOST 5272–68, does not include a corrosion resistance scale, nor is the term “steel with enhanced corrosion resistance” mentioned in other regulatory documents). The experience of their implementation has been mixed, and given their relatively high cost, they are not considered an optimal solution [29].
The optimal solution for protecting the inner surface of tubing from complicating factors is the use of various functional coatings [17; 30; 31]. Despite the wide variety of available coatings, in practice, only silicate-enamel (SEC), polymer, and duplex (intermetallic layer + polymer layer) coatings are commonly applied.
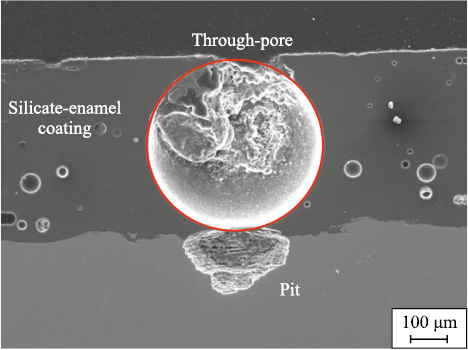
Silicate-enamel coatings are formed from a slurry prepared using frits of MK-5 and MK-5U grades with the following composition (wt. %): 0.5–3.5 Al2O3 ; 10.0–16.0 B2O3 ; 8.0–16.0 Na2O; 0.5–5.0 K2O; 2.0–5.0 Li2O; 2.0–8.0 CaO; 0.1–1.0 MgO; 3.0–6.0 TiO2 ; 0.5–5.0 MnO2 ; 0.3–2.0 NiO; 0.2–2.0 CuO; 0.3–1.5 CoO; 0.1–1.5 Fe2O3 ; and 0.5–4.0 F (in excess of 100 %). The coating can be applied using either a liquid or powder method, followed by firing at temperatures ranging from 850 to 950 °C. During the firing process, gases are released, which, upon cooling, result in the formation of porosity, often characterized by through-pores.
Despite their excellent resistance to ARPD formation, the use of tubing with silicate-enamel coatings (SEC) in the oil and gas industry has been declining, with the total volume not exceeding several thousand tons as of 2024. This reduction is attributed to several significant limitations:
– through-pore porosity in single-layer coatings (Fig. 3), which leads to severe pitting corrosion beneath the pores;
– high brittleness, which imposes restrictions on mechanical impacts and thread tightening torque; exceeding these limits can result in enamel chipping, particularly in the nipple area at the pipe ends;
– heat curing requirements at 850–950 °C, which prevent the use of heat-treated steel pipes, as such temperatures degrade their mechanical properties;
– higher costs compared to polymer coatings, primarily due to the need for high-temperature processing.
Fig. 3. Formation of pitting corrosion through the mechanism |
Advancements in polymer coatings, particularly the development of multifunctional coatings that combine anti-corrosion properties with resistance to ARPD formation, have largely negated the advantages of tubing with silicate-enamel coatings.
At present, the primary method for protecting the inner surface of tubing from complicating factors is the application of polymer coatings [32–35]. Their widespread adoption began in the early 2000s with the emergence of manufacturers such as MajorPack and Hilong. Although several production lines existed in Russia before this period, their products were not widely used. In the early stages of development, various film-forming coatings were applied, such as the polyurethane-based PolyPlex coating. However, it demonstrated an extremely short service life (typically less than 30 days), leading to its swift abandonment within the industry.
Polymer coatings offer several advantages over other protective methods against complicating factors. They provide extended service life for equipment and pipelines by protecting against corrosion and wear, significantly prolonging operational lifespan and reducing repair and replacement costs. Maintenance costs are also lower, as polymer coatings minimize the need for frequent servicing and repairs. Additionally, they enhance oil production and transportation efficiency by reducing friction, preventing deposits such as ARPD and inorganic salts, and improving thermal insulation, which leads to greater system performance. The risk of failures is reduced, as effective corrosion and wear protection helps prevent breakdowns associated with equipment deterioration. Furthermore, polymer coatings offer significant cost savings by extending service life and decreasing maintenance and repair expenses. Lastly, they provide environmental benefits by reducing emissions and leaks through improved corrosion resistance and enhanced equipment protection [36; 37].
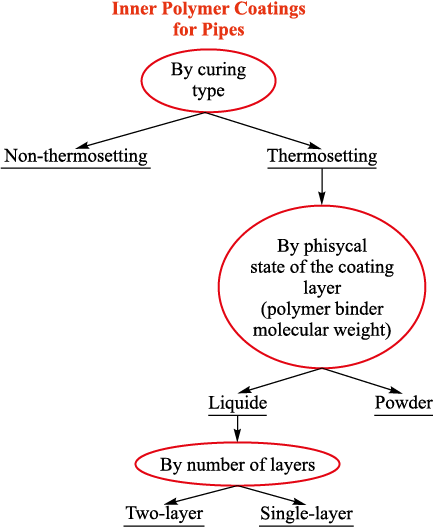
The classification of coatings used to protect the inner surface of tubing is shown in Fig. 4.
Fig. 4. Classification of polymer coatings used to protect |
There are several methods for applying internal polymer coatings, with the most commonly used being airless spraying and electrostatic application. The airless spraying method applies liquid coatings to the surface without the use of an air stream. Instead, high pressure forces the material through a nozzle, breaking it into fine droplets that uniformly coat the surface. This technique provides higher efficiency and better application quality compared to conventional air spraying and allows for the application of high-viscosity coatings with 100 % solids content. Electrostatic application, on the other hand, involves applying a layer of polymer powder to the product’s surface using an electrostatic field. In this process, the polymer particles are electrically charged and attracted to the surface, which has an opposite charge, ensuring a uniform and durable coating [38].
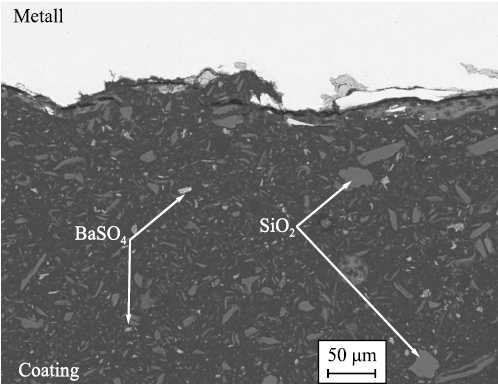
Initially, the effectiveness of internal coatings was questioned, as early applications utilized cold-cured epoxy resins based on Bisphenol A and epoxy novolac resin in an approximate 2:1 ratio (Fig. 5). The fillers and pigments commonly used in these coatings included micronized barite (BaSO4 ), aerosil (fumed silica, SiO2 ), titanium dioxide (TiO2 ), and talc (Mg3Si4O10(OH)2 ). Their concentrations varied depending on the formulation, but the total filler content typically did not exceed 50 %. These coatings exhibited relatively low glass transition temperatures (usually below 60 °C), limited chemical resistance, and restricted operating temperature ranges. They were applied using dual-component airless spray systems.
Fig. 5. Structure of a typical liquid non-thermosetting |
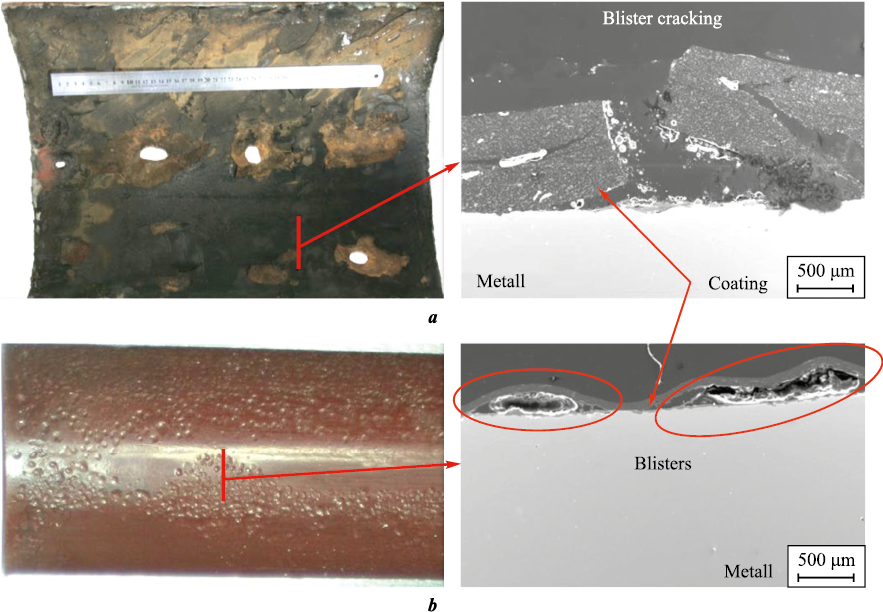
The degradation of pipes with the aforementioned type of coatings (Fig. 6), as evidenced by more than 40 expert studies conducted under the author’s supervision, highlights the low barrier properties of these coatings. The service life before the onset of blistering and delamination typically does not exceed 2–3 years for field pipelines. The use of such coatings for tubing protection is viable only at relatively low temperatures (up to 40 °C) and in cases where complications are limited to ARPD. Even under these conditions, their service life rarely exceeds 1 year.
Fig. 6. Degradation of liquid non-thermosetting internal epoxy coatings |
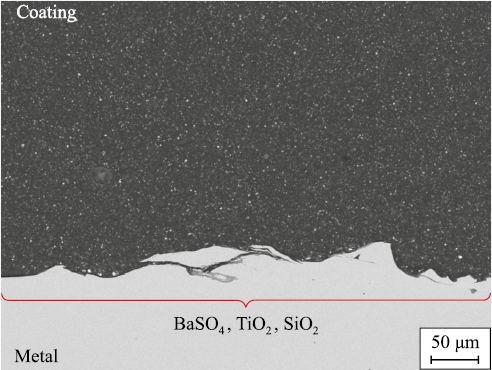
The liquid thermosetting epoxy novolac coating (its structure shown in Fig. 7) stands apart from other coatings due to its higher concentration of novolac resin, with an epoxy novolac resin to Bisphenol A-based resin ratio of ≥1:1, and the inclusion of a reactive diluent. This diluent lowers the viscosity of the epoxy composition, facilitating easier processing while also participating in the curing reaction and becoming an integral part of the polymer matrix. The application process is similar to that of non-thermosetting liquid epoxy coatings, with the key difference being the requirement for an isothermal curing hold at 180–200 °C for at least 20 min.
Fig. 7. Structure of a typical liquid thermosetting |
This type of coating is the most commonly used for tubing protection, as it delivers the required performance characteristics at relatively small thicknesses (~150 µm). It also enables the application of two-layer coatings, allowing for formulation optimization at smaller production facilities. The industry-wide use of these coatings is estimated to be 85–90 %.
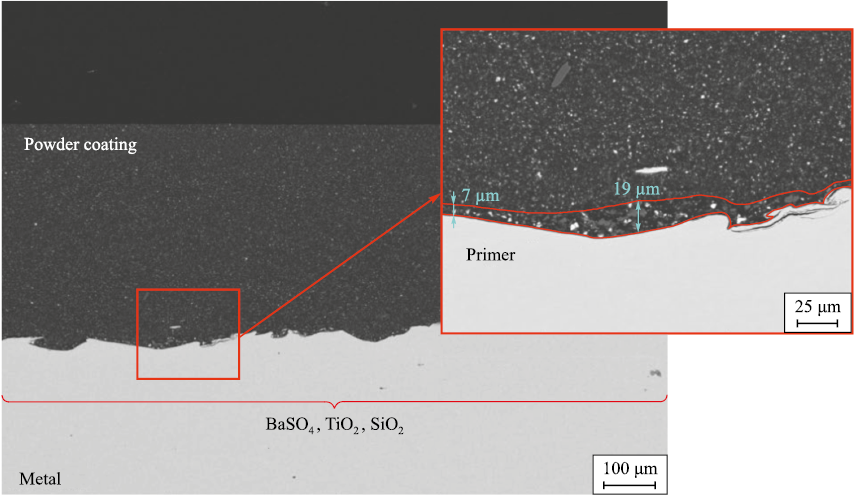
In the past three years, two-layer powder coatings have been increasingly adopted, consisting of a primer layer with a thickness of 5–40 µm and a top layer of ≥350 µm made from a powder composite material (Fig. 8). The primer is a paint-and-lacquer material composed of a blend of high-molecular-weight epoxy and phenol-formaldehyde resins, butyl cellosolve, toluene, and, in most cases, iron oxide pigment, which actively reacts with hydrogen sulfide during operation or testing. The powder coating is applied electrostatically to a preheated surface at a temperature of 160–200 °C. Its composition includes high-molecular-weight epoxy resin and a significant amount of fillers (up to 70 %), with a composition similar to those described earlier. This technology offers several advantages, including superior barrier properties, lower costs compared to other protective methods, high process efficiency and maintainability, and the ability to apply multifunctional coatings.
Fig. 8. Structure of a typical two-layer powder coating with an epoxy-phenolic primer |
Additionally, the absence of solvent evaporation during application makes this method more environmentally friendly and safer for production workers, which is why it is the only permitted option in most countries. The production volume of tubing with internal functional coatings is expected to exceed 100,000 tons by the end of 2024 (based on the weight of \(\emptyset \) 73×5.5 mm tubing) and continues to grow at a rate of 8–12 % per year.
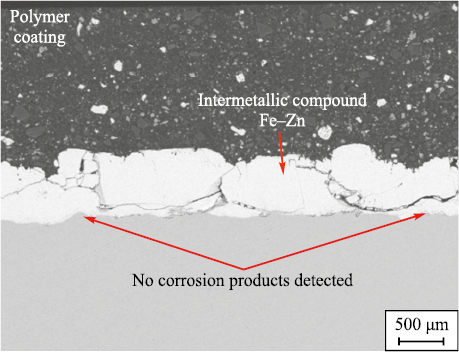
A relatively new technological solution is the use of duplex coatings, where the first layer consists of thermodiffusion zinc (TDZ), forming Fe–Zn intermetallic compounds, followed by one or more polymer layers [39]. These coatings demonstrate higher corrosion resistance in hydrogen sulfide-containing environments, as confirmed by laboratory autoclave exposure tests conducted over 2352 h, compared to the standard test duration of 240 h specified by GOST 58346–2019. The test involved exposing samples with two types of coatings in an autoclave under a partial gas pressure of H2S – 1 MPa, N2 – 9 MPa at a temperature of 80 °C. The results showed the presence of iron sulfide at the metal-coating interface in pipes without the TDZ layer, whereas no corrosion products were detected on duplex-coated pipes (Fig. 9). It should be noted that the autoclave testing methods used in this experiment and outlined in GOST 58346–2019 are based on previous research [40; 41]. The widespread adoption of autoclave testing methods has led to a significant increase in the service life of tubing with internal coatings. Although exact statistical data is not publicly available, sources accessible to the author indicate that the average service life has increased from 418 to 786 days. However, the disadvantages of duplex coatings include higher costs compared to conventional polymer-coated pipes and the limited availability of reliable solutions for protecting the external surface from corrosion.
Fig. 9. Surface condition of the metal–intermetallic layer (Fe–Zn) |
A key trend in the development of tubing with internal coatings is the creation of multifunctional coatings that combine anti-corrosion and anti-abrasion properties while also preventing ARPD and inorganic salt deposition [42–45]. Until recently, advancements in coating formulations and application technologies – such as the introduction of non-thermal microwave treatment [46] – were limited by the lack of standardized laboratory testing methods. The applicability of each coating was primarily determined through field pilot tests (FPT), which typically take about one year to complete.
To address the challenge of simulating ARPD formation under controlled conditions, two circulating test benchs were developed and manufactured under the author’s supervision [47; 48]. The test medium used in these test benches is an oil emulsion sampled from wells affected by ARPD, further enriched with deposits obtained during cleaning operations. The design of the test benches allows for adjustments in the composition of the test medium, flow rate, medium temperature, and the external surface temperature of the sample. The temperature difference across the inner surface of tubing samples – used as test specimens – facilitates the formation of deposits. The capabilities of these benches cover a wide range of well conditions, from low- to high-production wells, with varying temperature regimes influencing ARPD formation.
Research findings indicate that parameters such as surface roughness, paraffin adhesion to a dry surface, and the contact angle of distilled water on a dry surface do not provide a reliable assessment of a surface’s resistance to ARPD deposition. The laboratory method for determining the contact angle by measuring the spreading of an oil droplet in water on the coating surface has shown the highest correlation with test bench results [49]. The best ARPD resistance results were demonstrated by hydrophilic surfaces; however, they exhibited poor corrosion resistance. Therefore, the use of multifunctional coatings or two-layer systems is required to achieve balanced performance.
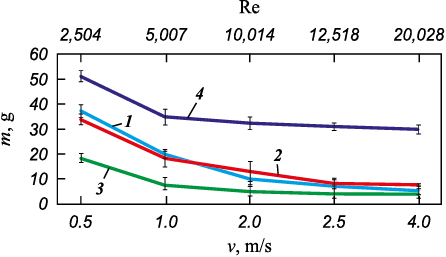
Experimental studies have established a correlation between ARPD deposition and flow rate (Reynolds number), allowing for the ranking of coatings based on their resistance to ARPD. Silicate-enamel coatings provide the highest resistance, followed by polymer coatings, while bare steel samples show the lowest performance (Fig. 10). The obtained results align with data from field pilot tests and the operational performance of these coating types across various oilfields.
Fig. 10. Dependence of asphaltene-resin-paraffin deposit mass on the flow rate |
Another important step in expanding the application of functional polymer coatings was the development of a test bench (Patent No. RU2825169C1) and a methodology to evaluate the effectiveness of coatings in preventing inorganic scale formation [50]. The goal of the test trials was to identify the coating with the highest resistance to scaling under oilfield conditions. The coatings’ resistance to scaling was assessed based on the mass of inorganic scale formed on the outer surfaces of cylindrical samples and the thickness of the resulting scale layerй.
The results of the test trials evaluating the resistance of coatings to gypsum-type (CaSO4 ) scale formation with halite (NaCl) impurities were published in [51]. It was found that none of the tested protective coatings could completely prevent the formation of gypsum scale with halite impurities on their surface. The study [51] also concluded that the adhesion strength of the “scale–coating” interface does not play a decisive role in the anti-scaling properties of protective coatings. The findings showed that scale deposits can form even on surfaces with minimal adhesion strength. These deposits are capable of creating solid structures with little to no interaction with the surface. Further analysis in [52] compared the results of the test trials [51] with the roughness parameters of the tested protective coatings to evaluate the effect of surface roughness on scaling. A certain correlation was observed between the coating’s roughness index and the mass of the scale layer formed on it. The steel sample with the highest surface roughness exhibited the most significant increase in the scale layer mass during dynamic tests. However, the relationship between surface roughness and scale formation mass was not strictly linear [52].
Since studies [51] and [53] both found that none of the examined coatings could completely prevent scale formation, further research focused on assessing the combined use of coatings with other preventive methods. In study [54], laboratory-scale dynamic tests were conducted to evaluate the feasibility of integrating internal protective tubing coatings with scale inhibitors. The findings showed that the combined approach can provide the following benefits:
– coated surfaces had fewer crystallization centers for inorganic scale deposits compared to uncoated steel surfaces;
– during testing (with a scale inhibitor dosage of 200 g/m3), the formed scale deposits detached more readily from the coated samples [54].
Coating of SEM and ESP housings
Various corrosion protection methods are employed to mitigate the corrosive impact and extend the service life of submersible electric motors (SEM) and electric submersible pumps (ESP). (Hereinafter, the discussion will focus solely on SEM housings; however, all conclusions are equally applicable to ESP housings.) One of the simplest and most cost-effective ways to enhance the service life of SEM housings while reducing exposure to aggressive factors under field conditions is the application of metallization coatings. Among the most widely used methods for applying such coatings are electric arc spraying (EAS ) and high-velocity oxy-fuel (HVOF) spraying [55–60].
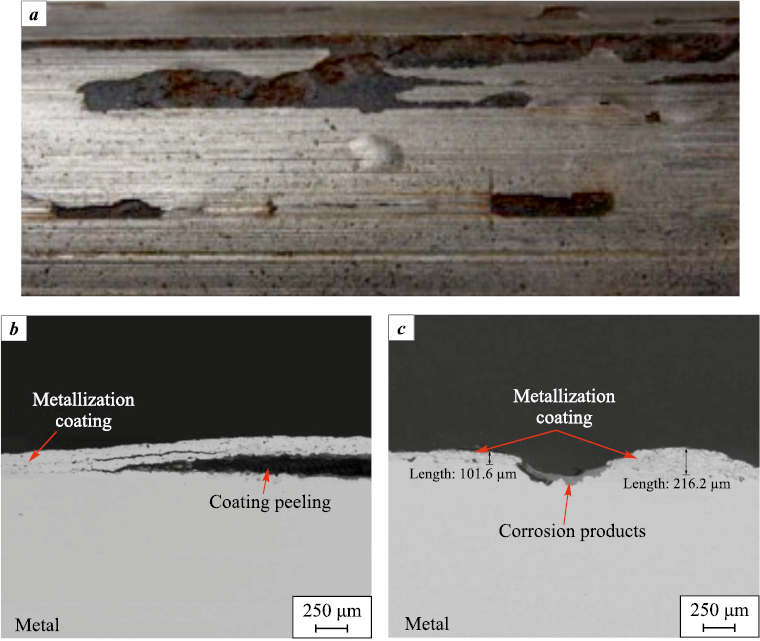
An analysis of the causes of SEM and ESP housing failures was conducted under the author’s supervision in four oil-producing regions with various complicating factors. The study revealed that corrosion of the SEM housing is the most common cause of failure [61–63]. The examination of SEM housings with metallization coatings applied using technologies implemented at pipe bases after operation allowed the identification of key causes of failure, including mechanical damage, abrasive wear of the coating, low barrier properties, and imperfections in the coating application technology (Fig. 11). Additionally, cases were identified where multiple negative factors were simultaneously present, making it impossible to determine the dominant failure mechanism. In many cases, these factors create a synergistic effect – for example, the simultaneous presence of a corrosive environment and abrasive particles results in corrosion-erosion wear, which occurs at a significantly higher rate compared to either corrosion or erosion alone [61].
Fig. 11. External view of the SEM housing with peeling and blistering |
The primary challenge in ensuring the operational reliability of SEM housings was the development of methodologies that simulate the effects of key complicating factors and conducting laboratory tests on various types of metallization coatings. The results, summarized in [61], onfirm the feasibility of accurately modeling the destructive impact of key complicating factors through autoclave tests in H2S- and CO2-containing environments [64; 65]. To determine the corrosion resistance of metallization coatings, samples must undergo autoclave exposure for 240 h. Testing typically involves the combined exposure to aggressive gases (CO2 , H2S). In order to isolate the corrosion effects of individual system components, tests can also be conducted in environments saturated with only carbon dioxide or hydrogen sulfide. To prevent decompression effects, pressure release must take at least 10 min [64].
Electric arc spraying (EAS) was selected as the primary coating application method due to its high process efficiency and equipment mobility. EAS coatings made of stainless steel materials based on iron and nickel were applied with a target thickness of 350–500 µm. The chemical composition of the metallization coatings included various elements in specific proportions (wt. %:
– Cr ~ 6.6÷14.5; Ni ~ 4.4÷8.4; Mo ~ 2.5; Si ~ 2.0÷3.7; Fe – balance;
– Cr ~ 13.0÷16.0; Ni ~ 7.3÷9.8; Mo ~ 3.5; Si ~ 2.9÷3.7; Al ~ 1.0; Fe – balance;
– Cr ~ 17.7÷18.6; Ni ~ 8.5÷8.9; Ti ~ 0.6; Si ~ 0.5÷0.7; Fe – balance.
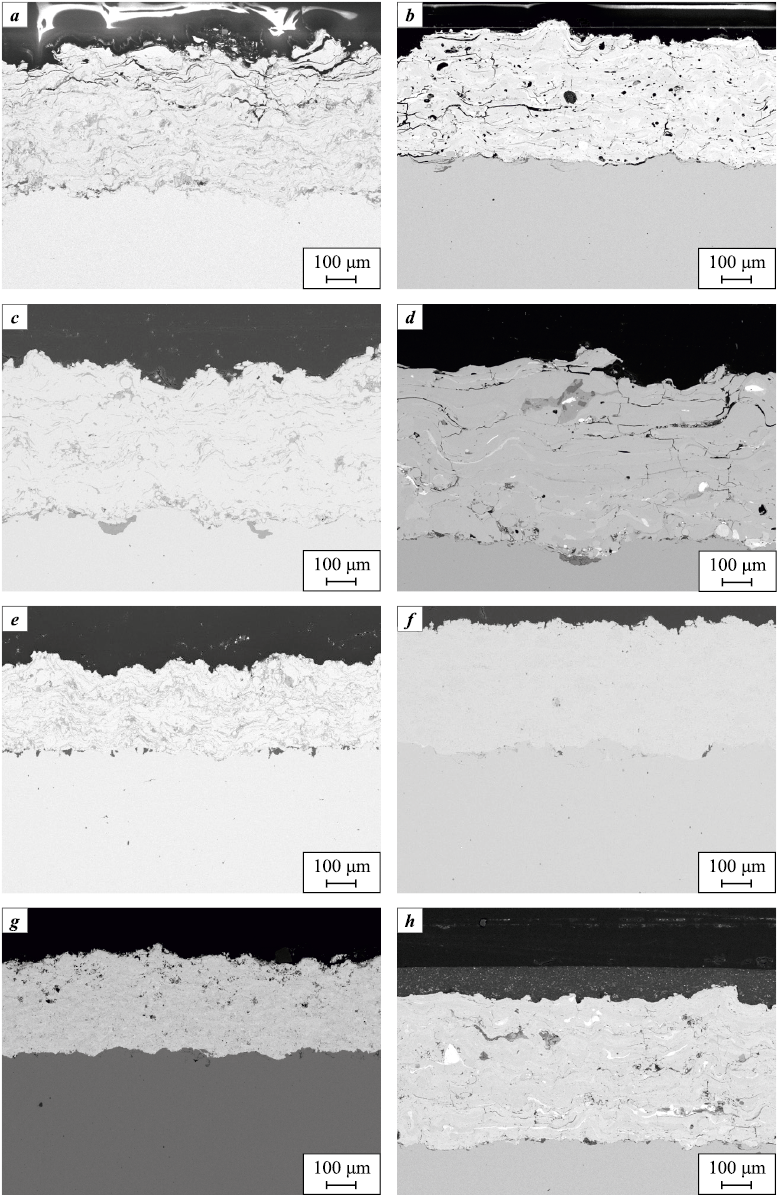
The physical and mechanical properties of EAS coatings were found to be low due to significant porosity and oxide layers between particles. Metallization coatings applied using iron-based wires (Fig. 12, a) without additional impregnation or an external polymer layer proved to be ineffective against corrosive environments. In contrast, nickel-based wire coatings (Fig. 12, b) demonstrated resistance in acidic environments but lacked sufficient protection against CO2- and H2S-saturated conditions, indicating their inability to provide long-term protection. To reduce particle oxidation during spraying, an argon protective atmosphere was used (Fig. 12, c, d), which resulted in a 55 % improvement in physical and mechanical properties compared to coatings applied in open air; however, corrosion resistance remained unsatisfactory [66].
Fig. 12. Microstructure of the coating |
Impregnation materials are commonly used to seal coating pores [67; 68]. The study utilized polymer-based impregnating materials, including epoxy-phenolic, acrylic, and polytetrafluoroethylene compositions with thicknesses of 70–150 µm. The application of impregnation materials improved the corrosion resistance of the metallization layer; however, even minor damage to the impregnated coating on iron-based layers resulted in rapid degradation. Therefore, the use of iron-based coatings with impregnation was deemed impractical.
To increase the spraying flame temperature and achieve greater particle melting, activated arc spraying (AAS) was employed in a propane-air environment [69]. The use of AAS with iron-based wires improved the physical and mechanical properties of the coating (Fig. 12, e). However, due to the combustion of the propane-air mixture during application, the metallization layer still contained oxide layers that were not resistant to aggressive environments, making AAS coatings unsuitable in their pure form. The application of an epoxy novolac resin-based impregnation helped to limit the access of corrosive media to the metallization layer, thus preventing its destruction. However, in areas with artificially introduced defects such as “scratches”, the metallization layer deteriorated, and corrosion products were observed on the substrate.
Using high-velocity oxy-fuel (HVOF) spraying, self-fluxing powder materials based on nickel (Fig. 12, f) and tungsten carbide in a cobalt matrix (Fig. 12, g) were applied with a thickness of 300–350 µm. These coatings demonstrated high physical and mechanical properties and sufficient resistance to corrosive environments. The HVOF-applied layer exhibited higher density with fewer oxide films between particles, which was achieved due to the higher particle velocity and shorter exposure time to the gas-oxidizing environment compared to EAS. The use of tungsten carbide-based powder materials provided coatings with excellent hardness and wear resistance; however, high porosity allowed aggressive media to penetrate and lead to substrate degradation.
Based on the results of the conducted studies, the following conditions were found to provide satisfactory outcomes:
– a nickel-based metallization layer containing ~18 % chromium and ~13 % molybdenum, applied using EAS, followed by impregnation with an epoxy novolac resin composition with a thickness of 70–150 µm;
– a nickel-based self-fluxing powder metallization layer containing ~16 % chromium, applied using HVOF spraying.
It is noteworthy that with comparable properties, the cost of the HVOF-applied coating is lower than that of EAS.
Conclusion
The introduction of anti-corrosion coatings for protecting submersible equipment used in oil production, particularly the inner surface of tubing, began in the late 1990s with the adoption of polymer coatings. The extension of service life was accompanied by advancements in coating formulations, transitioning from Bisphenol A-based epoxy resins to epoxy novolac resins, as well as improvements in application methods. These developments led to the introduction of thermal curing at temperatures of 170–200 °C. A significant milestone in this evolution was the development of two-layer systems that combined high barrier properties with resistance to ARPD and inorganic salt deposition. Currently, manufacturers of protective coatings face the challenge of developing multifunctional single-layer coatings that combine these protective capabilities.
At the same time, continuous improvements have been made to the composition and structure of metallization coatings. Traditional coatings applied using EAS with iron-based wires – characterized by overall porosity levels of up to 10 % and the presence of oxide films along particle boundaries – failed to provide adequate barrier protection. This resulted in the formation of corrosion products at the metal–coating interface, leading to rapid deterioration. Two parallel approaches have been pursued to enhance the operational reliability of such coatings: developing high-temperature polymer impregnations to seal porosity in the upper layers, and adopting HVOF spraying technology, which enables the production of non-porous, high-alloy corrosion-resistant coatings. While the scientific basis for these methods has been established, their full-scale implementation at production facilities is still required, along with efforts to replace imported components with domestically produced alternatives.
References
1. Banerjee S. Developments and challenges of mature oil fields. The Way Ahead. 2013;9(3):11–13. https://doi.org/10.2118/0313-011-TWA
2. Abdel-Basset M., Al-Mufarej M., Al-Mutawa M., Chetri H., Anthony E., Al-Zaabi H., Bolanos N., Ruiz H., Harami K. Integrated production optimization workflow provides robust platform for significant oil gain to a mature oilfield. In: Abu Dhabi International Petroleum Exhibition and Conference (Abu–Dhabi, UAE, 12–15 November 2018). 2018:D022S150R001. https://doi.org/10.2118/193121-MS
3. Stetsyuk I.A. Analysis of the effectiveness of various protection methods for CHO wells of the complicated fund of LLC “RN-Purneftegaz”. Oilfield Engineering. 2017;(8):40–43. (In Russ.).
4. Agafonov A.A., Nasedkin I.G., Novikova N.V., Buldakova N.S. Evaluation of the efficiency of using a magnetic inductor for combat cutting with ARPD on the production fund of PJSC “Udmurtneft”. Oilfield Engineering. 2020;(1):20–24. (In Russ.).
5. Mitroshin A.V., Dubovtsev A.S., Dulesova L.G. Analysis of complicating factors during mechanized oil production at PJSC “LUKOIL” enterprises. Geology, geophysics and development of oil and gas fields. 2019;(6):57–60. (In Russ.). https://doi.org/10.30713/2413-5011-2019-6(330)-57-60
6. Fajobi M.A., Loto R.T., Oluwole O.O. Corrosion in crude distillation overhead system: A review. Journal of Bio- and Tribo-Corrosion. 2019;5:67. https://doi.org/10.1007/s40735-019-0262-4
7. El-Meligi A.A. Corrosion preventive strategies as a crucial need for decreasing environmental pollution and saving economics. Recent Patents on Corrosion Science. 2010;2:22–33. https://doi.org/10.2174/1877610801002010022
8. Fayomi O.S.I., Akande I.G., Odigie S. Economic impact of corrosion in oil sectors and prevention: An overview. Journal of Physics: Conference Series. 2019;1378(2):022037. https://doi.org/10.1088/1742-6596/1378/2/022037
9. Hou B., Li X., Ma X., Du C., Zhang D., Zheng M., Xu W., Lu D., Ma F. The cost of corrosion in China. npj Materials Degradation. 2017;1:4. https://doi.org/10.1038/s41529-017-0005-2
10. Misra S., Baruah S., Singh K. Paraffin problems in crude oil production and transportation: A review. SPE Production & Operations. 1995;10(1):50–54. https://doi.org/10.2118/28181-PA
11. Kumar A. Perspectives of flow assurance problems in oil and gas production: A mini-review. Energy & Fuels. 2023;37(12):8142–8159. https://doi.org/10.1021/acs.energyfuels.3c00843
12. Sousa A.M., Ribeiro T.P., Pereira M.J., Matos H.A. Review of the economic and environmental impacts of producing waxy crude oils. Energies. 2023;16(1):120. https://doi.org/10.3390/en16010120
13. Gorodilova K.E. Silicate-enamel coating – an effective method of protecting pipelines from corrosion and asphalt-resin-paraffin deposits. Oilfield Engineering. 2020;(5-6):66–69. (In Russ.).
14. Markin A.N., Nizamov R.E., Sukhoverkhov S.V. Oilfield chemistry: A practical guide. Vladivostok: Dalnauka, 2011. 288 p. (In Russ.).
15. Latypov O.R. Operation of oil and gas equipment in aggressive environments. Ufa: Ufa State Petroleum Technological University, 2018. 151 p. (In Russ.).
16. Sousa A.L., Matos H.A., Guerreiro L.P. Preventing and removing wax deposition inside vertical wells: A review. Journal of Petroleum Exploration and Production Technology. 2019;9:2091–2107. https://doi.org/10.1007/s13202-019-0609-x
17. Yudin P.E., Petrov S.S., Maksimuk A.V., Knyazeva Zh.V., Prokudin A.V. Features of operation of pump and compressor pipes in conditions of wells of the corrosion fund. Korroziya. Territorii Neftegaz. 2018;40(2):50–54. (In Russ.).
18. Craig B.D. Evaluation and application of highly alloyed materials for corrosive oil production. Journal of Materials for Energy Systems. 1983;5(1):53–58.
19. Lasebikan B.A., Akisanya A.R., Deans W.F. The mechanical behavior of a 25Cr Super duplex stainless steel at elevated temperature. Journal of Materials Engineering and Performance. 2013;22:598–606.
20. Hahn B., Konrad J., Schneider A., Stallybrass C. Steel alloy for ferritic steel having excellent creep strength and oxidation resistance at elevated usage temperatures: Patent 9080230B2 (US). 2015. https://patents.google.com/patent/US9080230B2/en
21. Urech B.A., Stewart T., Fullerton P. System and method for producing bimetallic line pipe: Patent 2005/0251987A1 (US). 2005. https://patents.google.com/patent/US20050251987
22. Shugol A.A. Test results of austenitic steel liner as a corrosion protection system for tubing and line pipes. Territoriya Neftegaz. 2021;(11-12):22–27. https://elibrary.ru/rnwvne
23. Pavlov D.G., Kaunov A.S. Protective system for tubing DCS inoxinside. In: Actual problems of scientific knowledge. New technologies of the fuel and energy complex 2022: Materials of the VI International Scientific and Practical Conference (Tyumen, 22 April 2022). Ed. S.N. Nagaeva. Tyumen: Tyumen Industrial University, 2022. P. 134–138. (In Russ.). https://elibrary.ru/dlwikg
24. Bogatov N.A., Bogatov A.A., Salikhyanov D.R. Lined corrosion-resistant tubing. Steel. 2014;(11):86–88. (In Russ.). https://elibrary.ru/uinruv
25. McMillan J.S. Method of lining tubular members including rolling and crushing a liner: Patent 4923663 (US). 1990.
26. Krips H., Podhorsky M. Hydraulisches Aufweiten — ein neus Verfahren zur Befestigungen fur Rohren. VGB Kraftwerkstechnik. 1976;56(7):456–464. (In Germ.).
27. Vakili M., Koutník P., Kohout J. Addressing hydrogen sulfide corrosion in oil and gas industries: A sustainable perspective. Sustainability. 2024;16(4):1661. https://doi.org/10.3390/su16041661
28. Liu Z.Y., Wang X.Z., Liu R.K., Du C.W., Li X.G. Electrochemical and sulfide stress corrosion cracking behaviors of tubing steels in a H2S/CO2 annular environment. Journal of Materials Engineering and Performance. 2014;23:1279–1287. https://doi.org/10.1007/s11665-013-0855-x
29. Kim S.K. Results of operational tests of oilfield pipelines and tubing made of steels with increased corrosion resistance at the fields of Lukoil-Komi. Oilfield Engineering. 2011;(11):44–52. (In Russ.).
30. Cao J., Ma W., Huang W., Su Z., Zhu Y., Wang J. A novel inner wall coating-insulated oil pipeline for scale and wax prevention. Processes. 2023;11(7):1964. https://doi.org/10.3390/pr11071964
31. McKeen L.W., Hofmans J., Nelissen J. Engineered internal downhole coating solutions for corrosion & deposition control in downhole production tubulars. In: Abu Dhabi International Petroleum Exhibition and Conference (Abu Dhabi, UAE, 11–14 November 2012). 2012, Р. SPE-161204-MS. https://doi.org/10.2118/161204-MS
32. Protasov V.N. Theory and practice of using polymer coatings in equipment and structures of the oil and gas industry. Moscow: Nedra, 2007. 375 p. (In Russ.).
33. Lauer R.S. The use of high performance polymeric coatings to mitigate corrosion and deposit formation in pipeline applications. In: NACE Corrosion. 2007. Р. NACE-07028.
34. Zeng Q., Kang L., Fan J., Song L., Wan S., Liao B., Guo X. Durable superhydrophobic silica/epoxy resin coating for the enhanced corrosion protection of steel substrates in high salt and H2S environments. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022;654:130137. https://doi.org/10.1016/j.colsurfa.2022.130137
35. Sorensen P.A., Kiil S., Dam-Johansen K., Weinell C.E. Anticorrosive coatings: A review. Journal of Coatings Technology Research. 2009;6(2):135–176. https://doi.org/10.1007/s11998-008-9144-2
36. Gabdrafikov R.R. Increasing the inter-repair period of production wells using tubing with protective coatings in the fields of the Komi Republic. In: Lomonosov scientific readings of students, postgraduates, and young scientists-2022 (Arkhangelsk, 1–30 April 2012). Arkhangelsk: Northern (Arctic) Federal University n.a. M.V. Lomonosov, 2022. Р. 97–99. (In Russ.). https://elibrary.ru/ekhvbs
37. Askari M. Downhole corrosion inhibitors for oil and gas production–a review. Applied Surface Science Advances. 2021;6:100128. https://doi.org/10.1016/j.apsadv.2021.100128
38. Eds. I. Inamuddin, R. Boddula, M.I. Ahamed, A.M. Asiri. Polymers coatings: technology and applications. John Wiley & Sons, 2020. 480 p.
39. Proskurkin E.V., Gelovani V.A., Sonk A.N., Petrov I.V., Yarema I.P., Sukhomlin D.A. Zinc coatings as modern systems for pipe corrosion protection. Steel. 2018;(6): 32–37. (In Russ.).
40. Aleksandrov E.V., Yudin P.E., Knyazeva Zh.V. New method of autoclave test for express analysis of anticorrosive coatings. Pipeline transport: Theory and practice. 2015;49(3):3–11. (In Russ.).
41. Yudin P.E., Knyazeva Zh.V. Evaluation of the barrier properties of internal anti-corrosive coatings of oil pipelines and pump-compressor pipes using an autoclave test. Pipeline transport: Theory and practice. 2016;53(1):3–8. (In Russ.).
42. Bai J., Jin X., Wu J.T. Multifunctional anti-wax coatings for paraffin control in oil pipelines. Petroleum Science. 2019;16(3):619–631. https://doi.org/10.1007/s12182-019-0309-7
43. Liu S., Wang C., Liu S., Li K., Luo H., Fan W., Wang H. pH-responsive smart composite coating with active anticorrosion and efficient scale inhibition properties. Progress in Organic Coatings. 2022;170:106973. https://doi.org/10.1016/j.porgcoat.2022.106973
44. Liang B., Sun Y., Bai Z., Li H., Shi Y., Lin D., Wang H. A novel UV-curable amphiphobic coating with dynamic air-layer copolymer brush offering stable dual-functional anti-scaling and anti-corrosion properties. Progress in Organic Coatings. 2024;194:108607. https://doi.org/10.1016/j.porgcoat.2023.107700
45. Guo Y., Wang J., Su X., Tang C., Wang J. Anti-scaling and anti-corrosive study on waterborne composite coatings based on siloxane-g-polybutadiene incorporated with PTFE and nano-SiO2-EDTA. Journal of Coatings Technology and Research. 2024;(November):1–9. https://doi.org/10.1007/s11998-024-01011-5
46. Baranov N.A., Yudin P.E., Maksimuk A.V., Taratorin A.N., Zheldak M.V., Knyazeva Zh.V., Petrov S.S. Microwave installation for modifying polymer coatings of internal pipe surfaces: Patent 2710776 C1 (RF). 2020. (In Russ.). https://elibrary.ru/murfbl
47. Baranov N.A., Zheldak M.V., Makarov E.A., Yudin P.E., Maksimuk A.V., Petrov S.S., Trofimov I.S., Bogatov M.V. Laboratory research circulation stand for testing methods of counteracting sedimentation and corrosion processes in a tubing column: Patent 202556 (RF). 2021. (In Russ.).
48. Maksimuk A.V., Yudin P.E., Verevkin A.G., Zheldak M.V., Bogatov M.V., Berkov D.V., Krysina D.A., Vyazgin D.S., Ivanov A.V. Laboratory testing stand for simulating operating conditions inside a tubing column submerged in an oil well during testing of various methods of counteracting corrosion and sedimentation: Patent RU2802764C1 (RF). 2023. (In Russ.).
49. Bogatov M.V., Yudin P.E., Verevkin A.G., Berkov D.V. Influence of hydrophilicity and oleophobicity on asphalt-resin-paraffin deposits formation. Petroleum Engineering. 2022;20(6):114–123. (In Russ.). https://doi.org/10.17122/ngdelo-2022-6-114-123
50. Berkov D.V., Kostyuk I.I., Yudin P.E., Verevkin A.G. Development of a stand for evaluating the resistance of internal protective coatings of tubing to inorganic scaling. Petroleum Engineering. 2024;22(1):160–172. (In Russ.). https://doi.org/10.17122/ngdelo-2024-1-160-172
51. Berkov D.V., Kostyuk I.I., Yudin P.E., Verevkin A.G. Possibility assessment for using protective coatings and polymer materials on tubing to prevent inorganic scaling on the inner surface of pipes. International Review of Applied Sciences and Engineering. 2024. https://doi.org/10.1556/1848.2024.00819
52. Berkov D.V., Yudin P.E., Verevkin A.G. Investigation of the effect of roughness of internal protective coatings of tubing on their ability to prevent gypsum scaling. In: Modern prospects for the development of science, technology, and technologies: Collection of scientific articles of the 2nd International scientific and technical conference (Voronezh, 11 October 2024). Kursk: CJSC “University Book”, 2024. Р. 64–76. (In Russ.). https://doi.org/10.47581/2024.TM-07/Berkov-Denis-01
53. Heydrich M., Hammami A., Choudhary S., Mockel M., Ratulowski J. Impact of a novel coating on inorganic scale deposit growth and adhesion. In: Offshore Technology Conference (Houston, Texas, May 2019). 2019. Р. OTC-29218-MS. https://doi.org/10.4043/29218-MS
54. Berkov D.V., Kostyuk I.I., Yudin P.E., Verevkin A.G. Application of protective coatings and scale inhibitors for tubing to prevent scaling. Petroleum Engineering. 2024;22(5):113–126. (In Russ.). https://doi.org/10.17122/ngdelo-2024-5-113-126
55. Jabbarov Sh.N. Corrosion susceptibility of underground oil and gas production equipment. In: Exact science: Collection of articles of the XVIII International scientific conference “Technocongress” (17 December 2017). Kemerovo: Publ. “Pluton”, 2017. P. 3–7. (In Russ.).
56. Romanov V.S., Goldshtein V.G., Vasilyeva N.S. Statistical analysis of technological violations in the operation of submersible electric motors. Transactions of the Kola Science Center of the Russian Academy of Sciences. 2018; 3-16(9):114–121. (In Russ.).
57. Knyazeva Zh.V., Andriyanov D.I., Yudin P.E., Vasin R.A. Study of the mechanism of carbon dioxide corrosion of thermal spray metallization coatings used to protect submersible electric motors. Petroleum Engineering. 2023;21(5);168–181. (In Russ.). https://doi.org/10.17122/ngdelo-2023-5-168-181
58. Łatka L., Pawłowski L., Winnicki M., Sokołowski P., Małachowska A., Kozerski S. Review of functionally graded thermal sprayed coatings. Applied Sciences. 2020;10(15):5153. https://doi.org/10.3390/app10155153
59. Kumar P.A., Khurana V. A review – study of thermal spray coatings for corrosive wear. International Journal of Advance Research, Ideas and Innovations in Technology. 2016;2(3):1–6.
60. Nguyen V.T., Thu Q.L., Nguyen T.A., Ly Q.C., Thi L.P., Thi H.P., Mai T.D.T. Arc thermal spray NiCr20 alloy coating: fabrication, sealant, heat treatment, wear, and corrosion resistances. International Journal of Electrochemistry. 2019;2019(1):8796958. https://doi.org/10.1155/2019/8796958
61. Knyazeva Zh.V., Yudin P.E., Amosov A.P., Petrov S.S., Maksimuk A.V. Classification of causes of destruction of metallization coating of submersible electric motors during operation. Science intensive technologies in mechanical engineering. 2019;9(99):25–32. (In Russ.). https://doi.org/10.30987/article_5d2df0884cc457.62830322
62. Knyazeva Zh.V., Andriyanov D.I., Yudin P.E., Vasin R.A. Studies of wear resistance and mechanism of abrasive wear of thermal spray coatings used to protect submersible electric motors. Petroleum Engineering. 2023;21(1): 90–102. (In Russ.). https://doi.org/10.17122/ngdelo-2023-1-90-102
63. Dorofeeva A.G. Identification of causes of destruction of housings of submersible electric motors. In: New technologies – for the oil and gas region: Materials of the International scientific and practical conference (Tyumen, 16–20 May 2016). Tyumen: Tyumen Industrial University, 2016. P. 207–210. (In Russ.).
64. Knyazeva Zh.V., Yudin P.E. Autoclave tests of metallization coatings. In: Prospective development of science, engineering and technology: Collection of scientific articles of the 10th International scientific and practical conference (Kursk, 30 October 2020). Kursk: South-West State University, 2020. P. 74–78. (In Russ.). https://doi.org/10.47581/2020/30.10.2020/MTO53/1/018
65. Yudin P.E., Zheldak M.V., Petrov S.S., Aleksandrov E.V., Manakhov A.M. Laboratory autoclave: Patent 130878 (RF). 2013. (In Russ.).
66. Andriyanov D.I., Yudin P.E., Knyazeva Zh.V., Pozdeeva A.Yu., Amosov A.P. Influence of the spraying gas environment on the properties of thermal spray coatings. Petroleum Engineering. 2024;22(4):115–125. (In Russ.). https://doi.org/10.17122/ngdelo-2024-4-115-125
67. Pozdeeva A.Yu., Amosov A.P., Yudin P.E. Materials for impregnation of porous metallized gas-thermal anticorrosive coatings of submersible oilfield equipment. Part 1. Petroleum Engineering. 2024;22(2):156–164. (In Russ.). https://doi.org/10.17122/ngdelo-2024-2-156-164
68. Pozdeeva A.Yu., Amosov A.P., Yudin P.E. Materials for impregnation of porous metallized gas-thermal anticorrosive coatings of submersible oilfield equipment. Part 2. Petroleum Engineering. 2024;22(3):113–122. (In Russ.). https://doi.org/10.17122/ngdelo-2024-3-113-122
69. Korobov Yu.S. Analysis of the properties of gas-thermal coatings. Ekaterinburg: Publ. Ural University, 2016. 92 p. (In Russ.).
About the Author
P. E. YudinRussian Federation
Pavel E. Yudin – Cand. Sci. (Eng.), Associate Professor of the Department of metal science, powder metallurgy, nanomaterials of Samara State Technical University; Director of Science of Samara Scientific and Production Center, LLC
3B Garazhny Proezd, Samara 443022, Russia
133 Molodogvardeiskaya Str., Samara 443001, Russia
Review
For citations:
Yudin P.E. Functional coatings of submersible oilfield equipment for protection against corrosion, asphalt, resin, paraffin and salt deposits: Review. Powder Metallurgy аnd Functional Coatings (Izvestiya Vuzov. Poroshkovaya Metallurgiya i Funktsional'nye Pokrytiya). 2025;19(1):58-74. https://doi.org/10.17073/1997-308X-2025-1-58-74